
Update on Antibody-Dependent Enhancement (ADE) in SARS-CoV-2 Infection and Vaccination
Its Occurrence was Denied. Where Does the Science Stand Now, in July 2024? Look no Further, the Evidence is Now So Clear, Researchers are Looking for Ways to Mitigate SARS-CoV-2 mRNA Injected ADE.
Antibody-Dependent Enhancement (ADE) is a complex immunological phenomenon where virus-specific antibodies, rather than neutralizing the virus, enhance its entry into host cells and amplify its replication. ADE can occur in the context of natural infections and vaccinations, leading to heightened disease severity. This phenomenon has been observed in various viral infections, including respiratory syncytial virus (RSV), dengue, and HIV, and has been a subject of intense scrutiny in the context of SARS-CoV-2, the virus responsible for COVID-19.
The historical context of ADE provides critical insights into its mechanisms and implications. One notable example is the RSV vaccine trials in the 1960s. Vaccinated children who later encountered natural RSV infection experienced more severe respiratory illness compared to unvaccinated children. This was attributed to ADE, where vaccine-induced antibodies facilitated enhanced viral entry and immune activation (Kim et al., 1969). Understanding the diverse mechanisms of ADE, its implications for vaccine development, and its potential occurrence in SARS-CoV-2 infection is crucial. This comprehensive analysis aims to elucidate the different types of ADE, evidence of ADE in SARS-CoV-2, and the risks and propoosed mitigation strategies associated with ADE in COVID-19 vaccines.
ADE in Convalescent SARS-CoV-2 Patients
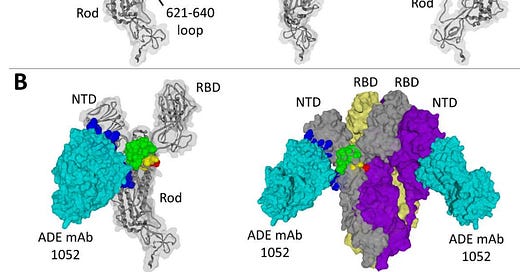
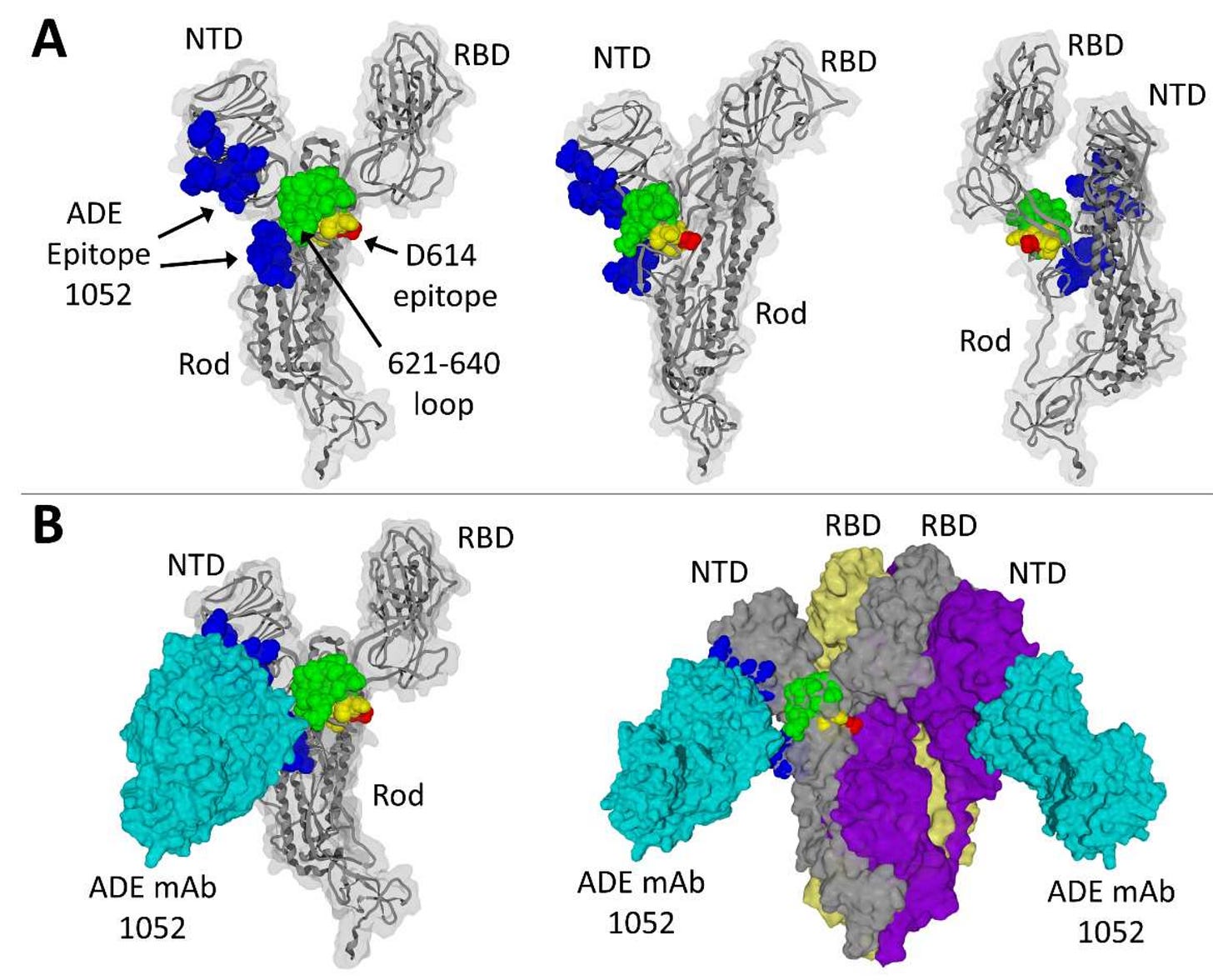
Guérin et al. (2022) reported that antibody-dependent enhancement (ADE) was observed in both convalescent COVID-19 patients and vaccinated individuals, especially with the Delta variant. This phenomenon involves antibodies facilitating viral entry into cells, potentially worsening the infection. The study indicates that ADE can occur due to the immune response targeting conserved and variable epitopes in the spike protein.
Fig 1. from Guérin et al. (2022)
The research is so refined the authors reported the mapped locations of “ADE epitopes”. These findings underscore the need for ongoing surveillance and evaluation of vaccine-induced immunity and potential risks associated with ADE in the context of emerging variants.
Types of ADE in SARS-CoV-2
It is now recognized that ADE can manifest through multiple pathways, primarily categorized based on the involvement of Fc receptors (FcRs) and the cellular responses they mediate. Nakayama and Shioda (2023) describe three primary types of ADE relevant to SARS-CoV-2:
Fc Receptor (FcR)-Dependent ADE of Infection in Macrophages
FcR-dependent ADE occurs when antibodies facilitate the virus's entry into Fc receptor-expressing cells, such as macrophages. These antibodies, which may have low neutralizing activity, bind to the virus and then interact with Fc receptors on the surface of macrophages. This interaction promotes the internalization of the virus-antibody complex, enhancing viral replication within the host cells (Nakayama & Shioda, 2023).
FcR-Independent ADE of Infection in Other Cells
In contrast, FcR-independent ADE does not involve traditional Fc receptor pathways. Instead, this type of ADE occurs in cells that do not express Fc receptors but may express other receptors or molecules that can interact with antibody-bound viruses. This mechanism is less understood but is crucial as it highlights the diversity of pathways through which ADE can occur (Nakayama & Shioda, 2023).
FcR-Dependent ADE of Cytokine Production in Macrophages
Another form of ADE involves the enhancement of cytokine production by macrophages upon Fc receptor interaction with antibody-virus complexes. This interaction can lead to an exaggerated immune response characterized by excessive cytokine release, commonly referred to as a "cytokine storm," which can cause significant tissue damage and exacerbate disease severity (Nakayama & Shioda, 2023).
ADE Phenomena Observed in SARS-CoV-2
In Vitro and In Vivo Evidence of ADE
Evidence of ADE in SARS-CoV-2 has been documented through various in vitro and in vivo studies. Nakayama and Shioda (2023) highlight that in vitro experiments have shown that antibodies from convalescent plasma can enhance SARS-CoV-2 infection in FcR-expressing cells. These findings suggest that non-neutralizing or sub-neutralizing levels of antibodies can facilitate viral entry and replication.
Wang et al. (2023) further corroborate these findings by demonstrating that the addition of human IgG to cell cultures can abolish ADE of SARS-CoV-2 infection. This suggests that specific antibody properties, such as affinity and concentration, play a critical role in mediating or preventing ADE (Wang et al., 2023).
Mechanisms Involving Non-Neutralizing Antibodies and Immune Response Dysregulation
The mechanisms underlying ADE involve non-neutralizing antibodies that bind to viral epitopes without effectively neutralizing the virus. These antibodies can form complexes with the virus, facilitating its entry into host cells through Fc receptor-mediated endocytosis. Once inside the cells, the virus can replicate more efficiently, leading to increased viral loads and disease severity (Nakayama & Shioda, 2023).
Additionally, these antibody-virus complexes can dysregulate the immune response, leading to an overproduction of pro-inflammatory cytokines. This cytokine dysregulation can cause tissue damage and exacerbate the clinical manifestations of the disease, as seen in severe COVID-19 cases (Wang et al., 2023).
Observations in Clinical and Epidemiological Studies
Clinical observations have reported cases where individuals with prior SARS-CoV-2 infections or vaccinations experienced more severe symptoms upon subsequent exposures. These observations align with the proposed mechanisms of ADE, where pre-existing antibodies might enhance viral entry or modulate the immune response in a deleterious manner. However, the exact incidence and clinical significance of ADE in SARS-CoV-2 infections remain subjects of ongoing research and debate.
ADE is So Accepted It is Now Impetus for Vaccine Development
Understanding the potential for ADE in SARS-CoV-2 is now considered critical for public health strategies and vaccine development. Barmada et al. (2023) argue that the risk of ADE from SARS-CoV-2 vaccination is such a serious risk that it necessitates careful consideration in vaccine design, particularly regarding antigen selection and antibody response modulation (see Lyons-Weiler, 2020: Pathogenic Priming et sub for the same argument). Current vaccines are designed to elicit a robust neutralizing antibody response to minimize the risk of ADE. Continuous monitoring and post-vaccination surveillance are essential to detect and mitigate any potential ADE-related complications (Barmada et al., 2023).
ADE and Vaccination
Antibody-Dependent Enhancement (ADE) poses significant concerns in the context of vaccine development, particularly for SARS-CoV-2. The phenomenon, where antibodies generated either from natural infection or vaccination enhance viral entry and replication, could potentially exacerbate disease severity. Next. we will review the risks associated with ADE from SARS-CoV-2 vaccines, evidence from clinical studies and proposed strategies to mitigate these risks.
Risks of ADE with SARS-CoV-2 Vaccines
The rapid development and deployment of SARS-CoV-2 vaccines have been a cornerstone in combating the COVID-19 pandemic. However, the potential for ADE has been a significant consideration during vaccine development. Vaccines aim to elicit a robust neutralizing antibody response to prevent infection. Still, there is a risk that they could also induce non-neutralizing antibodies capable of facilitating ADE.
Evidence from mRNA Vaccine-Associated Myocarditis
Recent studies have provided insights into potential ADE-related risks associated with mRNA vaccines, such as those developed by Pfizer-BioNTech and Moderna. Barmada et al. (2023) reported cases of myocarditis following mRNA vaccination, characterized by an aberrant immune response with cytotoxic lymphocytes and profibrotic myeloid cells. This cytokine-driven pathology suggests that ADE mechanisms could be at play, where vaccine-induced antibodies enhance inflammatory responses rather than neutralize the virus effectively (Barmada et al., 2023).
The concern extends to broader epidemiological observations. An article on Popular Rationalism highlights findings from a JAMA study that provide additional evidence of ADE, particularly focusing on the immune responses triggered by mRNA vaccines ([JAMA Article on ADE](https://popularrationalism.substack.com/p/jama-article-more-evidence-of-ade)). Another study suggests that both Moderna and Pfizer vaccines might be prone to ADE, indicating a need for ongoing surveillance and research to fully understand these dynamics ([Study on Moderna and Pfizer](https://popularrationalism.substack.com/p/study-suggests-that-moderna-and-pfizer)).
Anti-Glycan Antibodies (AGA) and Their Potential Role in ADE in SARS-CoV-2
Ziganshina et al. (2023) explored the role of anti-glycan antibodies (AGA) in ADE, particularly focusing on their presence in SARS-CoV-2 infections. AGAs, which target glycan structures on viral proteins, have been implicated in enhancing viral entry and replication. This phenomenon could occur if AGAs bind to viral glycoproteins, forming complexes that facilitate viral entry into host cells via alternative pathways. The study's findings suggest that individuals with higher AGA levels might be at an increased risk of ADE, underscoring the importance of considering glycan-specific immune responses in vaccine design (Ziganshina et al., 2023).
Mitigation Strategies
Given the potential risks of ADE, several strategies have been proposed to mitigate these effects in SARS-CoV-2 vaccines.
Addition of Human IgG to Abolish In Vitro ADE
Wang et al. (2023) demonstrated that the addition of human IgG to cell cultures could abolish ADE of SARS-CoV-2 infection in vitro. This approach suggests that enhancing the concentration of neutralizing antibodies could outcompete non-neutralizing ones, thereby preventing ADE. This finding underscores the importance of designing vaccines that elicit high titers of neutralizing antibodies to mitigate the risk of ADE (Wang et al., 2023; NB: Our inclusion of this research is not an endorsement.)
Fc-Silent Fusion Proteins and Their Role in Preventing ADE
Another promising strategy involves the use of Fc-silent fusion proteins in vaccine formulations. Honda-Okubo et al. (2023) explored the potential of Fc-silent proteins that lack the Fc receptor binding capability, thereby preventing Fc receptor-mediated ADE. These fusion proteins could provide reduce the risk of ADE by eliciting a strong neutralizing antibody response without the risk of enhancing viral entry via Fc receptors. Honda-Okubo et al., 2023 argue that this approach could be particularly beneficial in next-generation vaccines designed to minimize ADE risks while maintaining efficacy.
Case Studies and Reviews
To understand the implications and mechanisms of Antibody-Dependent Enhancement (ADE) in the context of SARS-CoV-2, it is critical to examine detailed case studies and comprehensive reviews. These studies provide insights into real-world occurrences of ADE, its clinical manifestations, and broader epidemiological patterns.
Myocarditis Post-mRNA Vaccination Showing Cytokine-Driven Pathology
One of the notable case studies involves myocarditis following mRNA vaccination, specifically the Pfizer-BioNTech and Moderna vaccines. Barmada et al. (2023) documented instances of myocarditis, highlighting a cytokine-driven pathology. This study reported an aberrant immune response characterized by cytotoxic lymphocytes and profibrotic myeloid cells. The authors suggest that this response could be indicative of ADE mechanisms, where vaccine-induced antibodies potentially enhance inflammatory responses rather than providing effective neutralization (Barmada et al., 2023). This case study underscores the need for ongoing vigilance and research to fully understand the immunological responses elicited by mRNA vaccines.
Review of ADE in Various Viral Infections and Potential Mechanisms in SARS-CoV-2
A comprehensive review by Sawant et al. (2023) explored the mechanisms of ADE across different viral infections, including dengue, HIV, and respiratory syncytial virus (RSV), drawing parallels to SARS-CoV-2. This review provided a detailed analysis of how non-neutralizing antibodies can facilitate viral entry and replication through Fc receptor pathways. The authors emphasized that ADE in SARS-CoV-2 could involve similar mechanisms, particularly in the presence of antibodies that bind to the virus without neutralizing it effectively. This review also highlighted the importance of understanding the role of different antibody types, such as IgG and IgM, in mediating ADE (Sawant et al., 2023).
Examples of ADE from Historical Viral Infections and Lessons for COVID-19 Vaccine Development
Historical examples of ADE in viral infections provide valuable lessons for the development of SARS-CoV-2 vaccines. For instance, the experience with RSV vaccines in the 1960s, where vaccinated children experienced more severe respiratory illness upon subsequent infection, underscores the potential risks of ADE. Sawant et al. (2023) argued that this knowledge informed the development of COVID-19 vaccines (a dubious claim), emphasizing the importance of eliciting a strong neutralizing antibody response to prevent ADE (a dubious conclusion).
Mechanistic Insights
Understanding the underlying mechanisms of ADE is essential for developing strategies to mitigate its risks. The interactions between antibodies, Fc receptors, and the complement system play crucial roles in mediating ADE.
Role of Fc Receptors and Complement System in ADE
Fc receptors, particularly FcγR, are critical in mediating ADE. Sawant et al. (2023) detailed how these receptors facilitate the entry of antibody-virus complexes into host cells, enhancing viral replication. FcγR-mediated ADE occurs when non-neutralizing antibodies bind to the virus and engage with FcγR on immune cells such as macrophages and dendritic cells. This interaction can facilitate cellular entry of the virus, leading to increased viral loads and inflammatory responses.
The complement system also plays a significant role in ADE. Complement proteins such as C1q and C3 can enhance viral infection by binding to antibody-virus complexes and facilitating their entry into host cells. Lusiany et al. (2023) discussed how the activation of the complement pathway could lead to the formation of immune complexes that promote viral entry and replication, contributing to ADE.
Impact of Somatic Mutations During Antibody Evolution on Neutralization and ADE
Somatic mutations during antibody evolution can significantly impact their neutralizing capacity and potential to mediate ADE. Wu et al. (2023) studied how somatic mutations in antibodies targeting the SARS-CoV-2 spike protein can either enhance or diminish their neutralizing ability. These mutations can lead to the production of antibodies with varying affinities for the spike protein, affecting their ability to prevent viral entry. Antibodies with lower neutralizing activity may enhance viral infection through ADE mechanisms, particularly if they facilitate Fc receptor-mediated entry into host cells (Wu et al., 2023).
Crosslinking of Spike Proteins by NTD-Targeting Antibodies Enhancing Viral Entry
Lusiany et al. (2023) examined the role of N-terminal domain (NTD)-targeting antibodies in enhancing viral entry. Their study found that antibodies binding to the NTD of the SARS-CoV-2 spike protein could crosslink adjacent spike proteins, facilitating viral entry into host cells. This crosslinking effect can potentiate ADE by increasing the efficiency of viral attachment and entry, particularly in Fc receptor-expressing cells. Understanding this mechanism highlights the importance of targeting the receptor-binding domain (RBD) rather than the NTD in vaccine design to minimize the risk of ADE (Lusiany et al., 2023).
The detailed case studies and mechanistic insights into ADE provide a comprehensive understanding of how this phenomenon can occur in the context of SARS-CoV-2 infection and vaccination. By examining real-world instances of ADE, reviewing its occurrence in other viral infections, and exploring the underlying molecular mechanisms, researchers and healthcare professionals can better anticipate and mitigate the risks associated with ADE. Continuous research and surveillance are essential to ensure the safety and efficacy of COVID-19 vaccines, ultimately contributing to more effective public health strategies.
Experimental Evidence
Understanding ADE's mechanisms and implications for SARS-CoV-2 involves extensive experimental research. This section explores the experimental evidence gathered from in vitro studies and animal models, highlighting key findings and their significance.
In Vitro Studies Demonstrating ADE with Specific Antibodies and Serum Samples
In vitro studies are essential for dissecting the molecular mechanisms of ADE. Wang et al. (2023) conducted experiments using serum samples from recovered COVID-19 patients and vaccinated individuals to study the potential for ADE. They observed that certain antibodies, particularly those with low neutralizing activity, could enhance SARS-CoV-2 infection in Fc receptor-expressing cells. This enhancement was evident when non-neutralizing antibodies facilitated viral entry into host cells, a key characteristic of ADE (Wang et al., 2023).
Furthermore, the study demonstrated that adding high concentrations of human IgG to the cultures could abolish this enhancement effect. This finding suggests that a strong neutralizing antibody response can counteract ADE, emphasizing the importance of vaccine formulations that elicit robust neutralizing antibodies (Wang et al., 2023).
Animal Model Studies Showing ADE Effects and Mitigation Strategies
Animal models provide a critical platform for studying ADE's in vivo effects and testing mitigation strategies. Early in the pandemic, studies were conducted on rhesus macaque monkeys using WIV-1 virus infection on WIV-1 mRNA injected animals. The conclusion of “no ADE” was published, and no further studies were conducted later on as new variants emerged and the wild-type virus evolved away from the vaccine type.
Thomas et al. (2024) investigated ADE in animal models exposed to SARS-CoV-2 antigens. Their studies involved mice and hamsters that were either naturally infected with SARS-CoV-2 or immunized with experimental vaccines. The findings revealed that certain conditions could trigger ADE, leading to increased viral loads and exacerbated disease symptoms in these models (Thomas et al., 2024).
To mitigate these effects, researchers experimented with different vaccine formulations and adjuvants. One approach involved using Fc-silent fusion proteins that lack the Fc receptor binding capability. Honda-Okubo et al. (2023) showed that these Fc-silent proteins could induce a strong immune response without the risk of ADE. In their hamster models, vaccines containing these proteins protected against heterologous infection with Beta or Delta variants, demonstrating their potential to enhance vaccine safety (Honda-Okubo et al., 2023).
These experimental studies underscore the importance of continuous research and development in vaccine design to prevent ADE. By understanding the conditions that lead to ADE and testing various strategies to mitigate these risks, scientists can develop safer and more effective vaccines.
Experimental evidence from in vitro and animal model studies provides crucial insights into the mechanisms of ADE and strategies to prevent it. These studies highlight the delicate balance required in vaccine design to elicit strong neutralizing antibody responses while minimizing the risk of ADE. As research progresses, the findings from these experiments will guide the development of next-generation vaccines that offer robust protection against SARS-CoV-2 and its variants without enhancing disease severity.
Controversies and Debates
The study of Antibody-Dependent Enhancement (ADE) in the context of SARS-CoV-2 has been fraught with controversies and debates. These stem from varying research findings, challenges in predicting and confirming ADE, and the implications of ADE for vaccine safety and efficacy. This section delves into these controversies, providing a balanced view of the ongoing scientific discourse.
Challenges in Predicting and Confirming ADE
One of the primary challenges in studying ADE is the difficulty in predicting and confirming its occurrence. ADE is a complex immunological phenomenon that can vary significantly based on the virus, particular host (hamster vs. rhesus macaques) immune response, and specific antibodies involved. Predicting ADE requires a deep understanding of these variables and their interactions, which is not always feasible.
Ziganshina et al. (2023) highlighted the lack of specific laboratory markers to definitively diagnose ADE. Without these markers, it is challenging to distinguish ADE from other immune-mediated disease exacerbations. This ambiguity complicates the interpretation of clinical data and hinders efforts to identify individuals at risk of ADE (Ziganshina et al., 2023).
Contradictory Findings in ADE Research
Research on ADE in SARS-CoV-2 has yielded contradictory findings, further fueling debates within the scientific community. Some studies have provided evidence supporting the occurrence of ADE, while others have found no significant indication of ADE in SARS-CoV-2 infections or vaccinations.
For instance, a meta-analysis by Gan et al. (2022) reviewed multiple studies on SARS-CoV-2 and found no conclusive evidence of ADE in the general population. The analysis included various observational studies and clinical trials, suggesting that while ADE is a theoretical risk, it may not be a prevalent phenomenon in SARS-CoV-2 infections or post-vaccination scenarios. The failures of the original animal models to detect ADE have been strongly attributed to the use of the wrong animal model and the use of the same virus type that induced the antibodies.
On the other hand, specific case studies and in vitro experiments have indicated the possibility of ADE. The findings by Barmada et al. (2023) on myocarditis following mRNA vaccination and the studies by Wang et al. (2023) on in vitro ADE highlight instances where ADE mechanisms could be at play. These discrepancies underscore the need for more robust, longitudinal studies to clarify ADE's role in SARS-CoV-2.
Need for Further Studies
Given the current uncertainties and contradictory findings, there is a consensus on the need for further studies to fully understand the implications of ADE. Longitudinal cohort studies, randomized controlled trials, and in-depth mechanistic studies are essential to elucidate ADE's prevalence, risk factors, and underlying mechanisms.
Ziganshina et al. (2023) and other researchers advocate for the development of specific biomarkers and diagnostic criteria to better identify and monitor ADE. These advancements could significantly enhance our ability to predict and mitigate ADE.
The controversies and debates surrounding ADE in SARS-CoV-2 highlight the complexities of this immunological phenomenon. While there is evidence supporting the potential for ADE, particularly in specific contexts, the overall prevalence and impact of ADE remain subjects of ongoing research. Addressing these controversies requires a concerted effort from the scientific community to conduct comprehensive studies, develop diagnostic tools, and continuously monitor vaccine safety.
Clinical Implications
Antibody-Dependent Enhancement (ADE) presents significant clinical implications, especially in the context of SARS-CoV-2 infection and vaccination. This section explores the potential impacts on vaccine design, deployment strategies, and clinical management practices, as well as the importance of monitoring and managing ADE in real-world settings.
Implications for Vaccine Design and Deployment
The risk of ADE has been a crucial consideration in the design and deployment of SARS-CoV-2 vaccines. It has been argued that vaccines aim to elicit a robust neutralizing antibody response while minimizing the production of non-neutralizing antibodies that could potentially enhance viral entry and replication. It has also been argued that understanding these dynamics is vital for developing safe and effective vaccines.
However, this view misses the original point made in early 2020 and every month thereafter to present: Exclusion of unsafe epitopes that induce these pathogenic responses in vaccines would be the wisest approach toward vaccine development that could not only avoid ADE, but also pathogenic priming and the subsequent development of autoimmunity and chronic illness post-vaccination.
Strategies to Avoid ADE in Vaccine Formulations
One of the primary strategies to mitigate the risk of ADE involves designing vaccines that elicit high titers of neutralizing antibodies. Honda-Okubo et al. (2023) discussed the use of Advax-CpG55.2-adjuvanted monovalent or trivalent SARS-CoV-2 recombinant spike protein vaccines, which have shown efficacy in protecting against heterologous infection in animal models. These vaccines aim to produce a strong neutralizing antibody response without enhancing viral entry through Fc receptor pathways (Honda-Okubo et al., 2023).
Another approach involves utilizing Fc-silent fusion proteins in vaccine formulations. These proteins lack the ability to bind to Fc receptors, thereby preventing Fc receptor-mediated ADE. This strategy is particularly relevant for next-generation vaccines designed to minimize ADE risk while maintaining high efficacy (Honda-Okubo et al., 2023).
Finally, exclusion of unsafe epitopes would prevent these issues altogether. This is clear to immunologists who study molecular mimicry and the strategey has been published many times over.
Monitoring and Managing ADE in Clinical Settings
Effective monitoring and management of ADE are essential to ensure vaccine safety and efficacy. Healthcare providers must be aware of the potential for ADE and be prepared to recognize and manage its clinical manifestations.
Case Reports and Clinical Observations of ADE in COVID-19 Patients
Clinical observations have reported cases where ADE may have contributed to severe disease outcomes. For instance, Matveev et al. (2023) described instances where antibodies capable of enhancing SARS-CoV-2 infection were detected in patients with severe COVID-19. These findings underscore the need for vigilance in monitoring immune responses and identifying potential ADE cases in vaccinated individuals and those with previous SARS-CoV-2 infections (Matveev et al., 2023).
Barmada et al. (2023) reported myocarditis following mRNA vaccination, characterized by an aberrant immune response. This cytokine-driven pathology suggests that ADE mechanisms could be involved, highlighting the importance of post-vaccination surveillance and clinical management strategies to mitigate adverse outcomes (Barmada et al., 2023).
The clinical implications of ADE in SARS-CoV-2 infection and vaccination are profound. Ensuring vaccine safety and efficacy requires a multifaceted approach, including designing vaccines that elicit robust neutralizing antibody responses, utilizing innovative vaccine formulations to prevent ADE, and maintaining vigilant monitoring and management practices in clinical settings. As research continues to evolve, these strategies will be crucial in safeguarding public health and optimizing vaccination programs.
Future Research Directions
As the scientific community continues to investigate the complexities of Antibody-Dependent Enhancement (ADE) in the context of SARS-CoV-2, several key areas for future research have emerged. These areas focus on developing diagnostic tools, enhancing vaccine formulations, and conducting long-term studies to better understand ADE and its implications. This section outlines these future research directions, emphasizing their importance in mitigating the risks associated with ADE and improving public health outcomes.
Identifying Specific Markers for ADE Prediction and Diagnosis
One of the most critical areas for future research is the identification of specific markers that can predict and diagnose ADE. Currently, the lack of definitive laboratory markers hampers efforts to distinguish ADE from other immune-mediated responses. Developing reliable biomarkers would enable healthcare providers to identify individuals at risk of ADE and monitor immune responses more effectively.
Kan and Li (2023) advocate for the development of specific biomarkers to enhance the prediction and diagnosis of ADE. These biomarkers could include specific antibody profiles, cytokine levels, and genetic markers that predispose individuals to ADE. By identifying these markers, researchers can develop targeted screening protocols and personalized interventions to prevent ADE (Kan & Li, 2023).
Developing Safer Vaccine Adjuvants and Formulations
Improving vaccine formulations to minimize the risk of ADE is another crucial research direction. Current vaccines are are not screened for unsafe epitopes, are not designed to elicit robust neutralizing antibody responses, but ongoing research is needed to optimize these formulations further and develop new adjuvants that enhance vaccine safety and efficacy.
Honda-Okubo et al. (2023) explored the use of Advax-CpG55.2 adjuvants in vaccine formulations, demonstrating their potential to induce strong neutralizing antibody responses without enhancing viral entry through Fc receptors. Future research should focus on developing and testing similar adjuvants that can provide effective immunity while mitigating ADE risks. Additionally, investigating the use of Fc-silent fusion proteins and other innovative approaches in vaccine design can contribute to safer vaccines (Honda-Okubo et al., 2023).
Longitudinal Studies to Assess the Long-Term Effects of Vaccination and Natural Infection on ADE
Longitudinal studies are essential to understand the long-term effects of vaccination and natural infection on ADE. These studies should track individuals over extended periods, monitoring immune responses, antibody profiles, and clinical outcomes to identify any potential ADE-related complications.
Kan and Li (2023) emphasize the importance of conducting longitudinal cohort studies to assess the durability of immune protection and the potential for ADE over time. Such studies can provide valuable insights into the dynamics of immune responses and help identify any delayed ADE effects. This research is critical for informing vaccination strategies and ensuring long-term vaccine safety (Kan & Li, 2023).
Investigating the Role of Non-Neutralizing Antibodies in ADE
Once unsafe epitopes are removed, further research is needed to elucidate the role of non-neutralizing antibodies in ADE. Understanding how these antibodies interact with viral epitopes and Fc receptors can provide insights into the mechanisms driving ADE and help develop strategies to mitigate its effects.
Wu et al. (2023) highlighted the impact of somatic mutations during antibody evolution on neutralizing and non-neutralizing antibodies. Future studies should investigate how these mutations influence antibody binding and ADE risk. By characterizing the properties of non-neutralizing antibodies and their interactions with the virus, researchers can develop targeted interventions to prevent ADE (Wu et al., 2023).
The future research directions outlined in this section highlight the importance of continued investigation into ADE and its implications for SARS-CoV-2 infection and vaccination. By developing diagnostic tools, improving vaccine formulations, conducting longitudinal studies, and investigating the role of non-neutralizing antibodies, the scientific community can better understand and mitigate the risks associated with ADE. These efforts are crucial for ensuring the safety and efficacy of vaccines and optimizing public health strategies in the ongoing fight against COVID-19.
References
Guérin, V.M.; Silva, R.D.; Fernandes, C.C.; Li, C.; Monteiro, T.F.; Monteiro, G.A.; Prazeres, D.M.F. (2022). Structural Dynamics of the SARS-CoV-2 Spike Protein: A 2-Year Retrospective Analysis of SARS-CoV-2 Variants (from Alpha to Omicron) Reveals an Early Divergence between Conserved and Variable Epitopes. Molecules, 27(12), 3851. https://doi.org/10.3390/molecules27123851
Nakayama EE, Shioda T. "SARS-CoV-2 Related Antibody-Dependent Enhancement Phenomena In Vitro and In Vivo." Microorganisms. 2023 Apr 13;11(4):1015. doi: [10.3390/microorganisms11041015](https://www.mdpi.com/2076-2607/11/4/1015).
Gan, L., Chen, Y., Tan, J. et al. Does potential antibody-dependent enhancement occur during SARS-CoV-2 infection after natural infection or vaccination? A meta-analysis. BMC Infect Dis 22, 742 (2022). https://doi.org/10.1186/s12879-022-07735-2
Wang X, Li M, Lu P, et al. "In Vitro Antibody-Dependent Enhancement of SARS-CoV-2 Infection Could Be Abolished by Adding Human IgG." Pathogens. 2023 Aug 30;12(9):1108. doi: [10.3390/pathogens12091108](https://www.mdpi.com/2076-2607/11/4/1015).
Barmada A, Klein J, Ramaswamy A, et al. "Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine-associated myocarditis." Sci Immunol. 2023 May 12;8(83):eadh3455. doi: 10.1126/sciimmunol.adh3455.
Ziganshina MM, Shilova NV, Khalturina EO, Dolgushina NV, Borisevich SV, Yarotskaya EL, Bovin NV, Sukh
MM, et al. "Antibody-Dependent Enhancement with a Focus on SARS-CoV-2 and Anti-Glycan Antibodies." Viruses. 2023 Jul 20;15(7):1584. doi: [10.3390/v15071584](https://www.mdpi.com/1999-4915/15/7/1584).
Honda-Okubo Y, Bowen R, Barker M, Bielefeldt-Ohmann H, Petrovsky N. "Advax-CpG55.2-adjuvanted monovalent or trivalent SARS-CoV-2 recombinant spike protein vaccine protects hamsters against heterologous infection with Beta or Delta variants." Vaccine. 2023 Nov 22;41(48):7116-7128. doi: 10.1016/j.vaccine.2023.10.018.
Matveev A, Pyankov O, Khlusevich Y, et al. "Antibodies Capable of Enhancing SARS-CoV-2 Infection Can Circulate in Patients with Severe COVID-19." Int J Mol Sci. 2023 Jun 28;24(13):10799. doi: 10.3390/ijms241310799.
Wu J, Chen Z, Gao Y, et al. "Fortuitous somatic mutations during antibody evolution endow broad neutralization against SARS-CoV-2 Omicron variants." Cell Rep. 2023 May 30;42(5):112503. doi: 10.1016/j.celrep.2023.112503.
Kan AKC, Li PH. "Inactivated COVID-19 vaccines: potential concerns of antibody-dependent enhancement and original antigenic sin." Immunol Lett. 2023 Jul;259:21-23. doi: 10.1016/j.imlet.2023.05.007.
Popular Rationalism. "JAMA Article: More Evidence of ADE." Popular Rationalism. Available online: (https://popularrationalism.substack.com/p/jama-article-more-evidence-of-ade).
Popular Rationalism. "Study Suggests that Moderna and Pfizer Vaccines may be Prone to ADE." Popular Rationalism. Available online: (https://popularrationalism.substack.com/p/study-suggests-that-moderna-and-pfizer).
Kim, H.W., et al. "Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine." American Journal of Epidemiology. 1969; 89(4): 422-434. doi: 10.1093/oxfordjournals.aje.a120955.












Well done. Thank you.
All vaccines are unneccessary and dangerous with all those toxic adjuvants. Shocking findings, known about but ignored by regulators. francesleader.substack.com/p/white-fibrous-clots Also rumble.com/user/DOCTORSEGALLA Chemists seem to have a much better grasp of the words "first do no harm."