Vaccine makers do not use placebos to test the safety of their vaccines for kids
Don't fall for semantic arguments: Inert placebos alone allow full undestanding of the risks associated with vaccines and their ingredients.
Below is a re-publication of the chapter Robert F. Kennedy, Jr. mentioned in the town hall. (ORIGINAL SOURCE: Children’s Health Defense).
“Vaccine makers do not use placebos to test the safety of their vaccines for kids
What is a Placebo?
A placebo is defined as a substance or treatment which is designed to have no therapeutic value. Common placebos include inert tablets (like sugar pills) and inert injections (like saline).
Here is a sampling of some pediatric vaccines. You will note that almost without exception, sugar pills, saline or other inert substances are not used.
Hep B Vaccine Tests
In this “safety study”, hepatitis B vaccines were compared to each other and not to a true placebo.
“Healthy infants were immunized at 2, 4 and 6 months of age with hepatitis B vaccine manufactured by either SmithKline Beecham (Engerix-B, 10 micrograms/dose, n = 228) or Merck and Co. (Recombivax HB, 2.5 micrograms/dose, n = 200).”
Comparative safety and immunogenicity of two recombinant hepatitis B vaccines given to infants at two, four and six months of age. D P Greenberg , et al., Pediatr Infect Dis J. 1996 Jul;15(7):590-6.
In this safety study “urban youth” received either a hepatitis B vaccine or a hepatitis B+A vaccine. No placebo.
“Urban youth, ages 12 to 17 years, at participating Adolescent Medicine Trials Network for HIV/AIDS Interventions Clinical/Research sites were randomized to receive either 2 doses of Recombivax HB (10 microg hepatitis B surface antigen) or Twinrix (20 microg hepatitis B surface antigen and 720 EL.U hepatitis A antigen) at 0 and 24 weeks.”
Randomized trial to determine safety and immunogenicity of two strategies for hepatitis B vaccination in healthy urban adolescents in the United States. Coleen K Cunningham, et al., Pediatr Infect Dis J. 2010 Jun;29(6):530-4.
Hib/Hep B Vaccine Tests
In this “safety study”, infants received either Hib vaccine with tetanus or hepatitis B plus DTP. They called the hepatitis B vaccine the control. No placebo.
“Infants were randomized to receive either Haemophilus influenzae capsular polysaccharide tetanus conjugate vaccine or a recombinant hepatitis B control vaccine (in addition to DTP) at approximately 2, 4, and 6 months of age.”
Effectiveness and safety of an Haemophilus influenzae type b conjugate vaccine (PRP-T) in young infants. Kaiser-UCLA Vaccine Study Group. C M Vadheim, et al., Pediatrics. 1993 Aug;92(2):272-9.
In this “safety study” of the Hib vaccine, kids received either Comvax (a Hib vaccine) or PedvaxHib (another Hib vaccine) plus Recombivax Hb (a hepatits B vaccine). No placebo.
“Eight hundred eighty-two healthy infants, approximately 2 months of age, were enrolled in an open, multicenter (n = 11) clinical trial and randomized to receive either COMVAX (7.5 micrograms of PRP/5 micrograms of HBsAg in 0.5 ml) or concurrent injections of the liquid formulation of PedvaxHIB (P) (7.5 micrograms of PRP in 0.5 ml) and RECOMBIVAX HB (R) (5 micrograms of HBsAg in 0.5 ml) at 2, 4 and 12 or 15 months of age.”
Safety and immunogenicity of a bivalent Haemophilus influenzae type b/hepatitis B vaccine in healthy infants. Hib-HB Vaccine Study Group. D J West, et al., Pediatr Infect Dis J. 1997 Jun;16(6):593-9.
In this “safety study”, infants received either a Hib vaccine or Hib vaccine with diphtheria. No placebo.
“To evaluate the safety and immunogenicity of the Haemophilus influenzae type b polysaccharide vaccine, PRP, and a new polysaccharide-diphtheria toxoid conjugate vaccine, PRP-D, a collaborative study was carried out in six centers in five states. Subjects were 585 infants 15 to 24 months of age. They were randomly assigned to receive a single dose of PRP or PRP-D vaccine.”
Safety and immunogenicity of Haemophilus influenzae type b polysaccharide and polysaccharide diphtheria toxoid conjugate vaccines in children 15 to 24 months of age. C D Berkowitz, et al., Pediatr. 1987 Apr;110(4):509-14.
DTP Vaccine Tests
In this “safety study”, acellular DTP was compared to cellular DTP. No placebo.
“An acellular pertussis vaccine principally containing two purified pertussis antigens, filamentous hemagglutinin and lymphocytosis-promoting factor, combined with diphtheria and tetanus toxoids was compared to conventional diphtheria-tetanus toxoids-whole cell pertussis for adverse effects and serologic responses in a group of 120 children who were from 18 to 24 months of age.”
Acellular pertussis vaccine: immunogenicity and safety of an acellular pertussis vs. a whole cell pertussis vaccine combined with diphtheria and tetanus toxoids as a booster in 18- to 24-month old children. M E Pichichero, et al., Pediatr Infect Dis J. 1987 Apr;6(4):352-63.
In this “safety study”, MMR+DPT was compared to DPT after MMR. No placebo.
“The safety and efficacy of simultaneous administration of measles-mumps-rubella (MMR), diphtheria-tetanus-pertussis (DTP), and trivalent oral poliovirus (OPV) vaccines in a test group of 405 children were compared with the safety and efficacy of sequential administration of the same vaccines in a control group of 410 children given MMR followed by booster doses of DTP and OPV 2 months later.”
Simultaneous administration of measles-mumps-rubella vaccine with booster doses of diphtheria-tetanus-pertussis and poliovirus vaccines. A Deforest, et al., Pediatrics. 1988 Feb;81(2):237-46.
In this safety study, whole-cell DPT was compared against acellular DPT. No placebo.
“Children were randomly assigned to receive either whole-cell diphtheria and tetanus toxoids and pertussis vaccine or one of three lots of acellular diphtheria and tetanus toxoids and pertussis vaccine (DT-aP) in a 1:3 ratio at the 11 private practices and in a 1:1 ratio at the university-affiliated practice and inner-city university pediatric clinic.”
Comparison of the safety and immunogenicity of acellular (BIKEN) and whole-cell pertussis vaccines in 15- to 20-month-old children. J F Marcinak, et al., Am J Dis Child. 1993 Mar;147(3):290-4.
Rotavirus Vaccine Tests
In this safety study, “inner-city infants” received either a rotarvirus vaccine (at two different concentrations) or “placebo.” The “placebo” was lyophilized uninfected tissue culture fluid. Arguably, this could be a true placebo because it may have had no therapeutic effect. But, nowhere in the study is the non-therapeutic effect of lyophilized uninfected tissue culture fluid referenced.
“To assess the safety and immunogenicity of bovine rotavirus vaccine, we administered attenuated strain RIT 4237 to 54 inner-city infants randomized to one of three groups in a double-blind fashion to receive a dose at 3 and 5 months of age of either placebo, vaccine virus at 10(7) TCID50/ml, or vaccine virus at 10(8) TCID50/ml.”
Safety and immunogenicity of bovine rotavirus vaccine RIT 4237 in 3-month-old infants. Y Maldonado, et al., J Pediatr. 1986 Dec;109(6):931-5.
In this safety study, Navajo infants received either a rotarvirus vaccine made from cows or a rotavirus vaccine made from monkeys (at two different concentrations) or “placebo.” The “placebo” is described as “placebo recipients were given 1 ml of Nursoy. Before administration of the vaccine or placebo, 30 ml of Nursoy buffered with 400 mg of sodium bicarbonate was given orally.”
So those receiving the placebo received Nursoy and sodium biocarbonate. Nursoy is a soy based infant formula. Sodium biocarbonate is commonly known as baking soda. Since sodium bicarbonate reduces stomach acid and is used as an antacid to treat heartburn, indigestion, and upset stomach, it does have a therapeutic effect. Therefore, it is not a true placebo. Interestingly, the children who received this “placebo” and did not get an actual vaccine had fewer hospitalizations. Rotarvirus vaccine is supposed to stop diarrhea. The infants who received the rhesus rotavirus vaccine had 2 hospitalizations for diarrhea (2/108 infants). The infants who received the bovine rotavirus vaccine had 3 hospitalizations from diarrhea (3/106 infants). The “placebo” had zero hospitalizations (0/107) infants.
“A double-blind, randomized, placebo-controlled trial was conducted to evaluate the safety and efficacy of a rhesus rotavirus vaccine and RIT 4237, a bovine rotavirus vaccine, in a Navajo population. Infants aged 2-5 months were randomized to receive one dose of either 10(4) pfu of the rhesus rotavirus vaccine or 10(8) pfu of the RIT 4237 vaccine or placebo. Before administration of the vaccine or placebo, 30 ml of Nursoy buffered with 400mg of sodium bicarbonate was given orally.”
A field study of the safety and efficacy of two candidate rotavirus vaccines in a Native American population. M Santosham, et al., J Infect Dis. 1991 Mar;163(3):483-7.
In this safety study, infants received one of two formulations of a rhesus monkey based rotavirus vaccine or “placebo.” “Placebo” consisted of tissue culture medium manufactured and coded by Wyeth-Ayerst Laboratories (the company that owns the vaccine). The study did not describe what this tissue culture medium was.
“In this randomized, double-blind trial, 1278 healthy infants ages 5 to 25 weeks received three oral doses of RRV serotype 1, RRV-TV, or a placebo at approximately 2, 4, and 6 months of age. Vaccines contained 4 x 10(5) plaque-forming units of virus.”
Safety and efficacy of high-dose rhesus-human reassortant rotavirus vaccines–report of the National Multicenter Trial. United States Rotavirus Vaccine Efficacy Group. M B Rennels, et al., Pediatrics. 1996 Jan;97(1):7-13.
Pneumococcal Vaccine Tests
In this “safety study”, young children received either one of three experimental Streptococcus vaccines or the licensed pneumococcal vaccine. No placebo.
“118 young children 18 to 30 months of age received a single immunization with one of the three glycoconjugates or with licensed pneumococcal vaccine.”
A randomized comparison of three bivalent Streptococcus pneumoniae glycoprotein conjugate vaccines in young children: effect of polysaccharide size and linkage characteristics. M C Steinhoff, et al., Pediatr Infect Dis J. 1994 May;13(5):368-72.
In this “safety study”, infants in Gambia were given either an experimental pneumococcal vaccine or a Hib type B vaccine. They called the kids who got the Hib Vaccine “controls.” There was no placebo.
“Thirty Gambian infants were vaccinated with 3 doses of a five-valent pneumococcal conjugate vaccine containing 5 micrograms of type 6B, 14, 18, 19F and 23F polysaccharides conjugated to the diphtheria toxin mutant protein CRM197 at the ages of 2, 3 and 4 months; 30 infants received 2 doses at the ages of 2 and 4 months and 30 infants who received three doses of a Haemophilus influenzae type b vaccine acted as controls.”
Pilot trial of a pentavalent pneumococcal polysaccharide/protein conjugate vaccine in Gambian infants. A Leach, et al., Pediatr Infect Dis J. 1996 Apr;15(4):333-9. doi: 10.1097/00006454-199604000-00010.
In this “safety study”, two-month old infants received either an experimental pneumococcal vaccine or a meningococcal vaccine. No placebo.
“Two hundred twelve healthy 2-month-old infants were equally randomized to receive four consecutive doses of PNCRM7 or an investigational meningococcal group C conjugate vaccine, which served as a control.”
Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. M B Rennels et al., Pediatrics. 1998 Apr;101(4 Pt 1):604-11.
Like the one above, in this “safety study”, children received either an experimental pneumococcal vaccine or a meningococcal vaccine. No placebo.
“The Wyeth Lederle Heptavalent CRM197 (PCV) was given to infants at 2, 4, 6 and 12 to 15 months of age in a double blind trial; 37,868 children were randomly assigned 1:1 to receive either the pneumococcal conjugate vaccine or meningococcus type C CRM197 conjugate.”
Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. S Black, et al., Pediatr Infect Dis J. 2000 Mar;19(3):187-95.
In this safety study, Gambian infants received either an experimental pneumococcal vaccine or polio vaccine. No placebo.
“Two hundred seven infants were randomized to receive three doses of either nonavalent protein conjugate pneumococcal vaccine (PnCV) or inactivated polio vaccine (IPV) at 2, 3 and 4 months of age with routine Expanded Program of Immunization vaccines as scheduled.”
Safety and immunogenicity of a nonavalent pneumococcal vaccine conjugated to CRM197 administered simultaneously but in a separate syringe with diphtheria, tetanus and pertussis vaccines in Gambian infants. S K Obaro, et al., Pediatr Infect Dis J. 2000 May;19(5):463-9. doi: 10.1097/00006454-200005000-00014.
Varicella (Chickenpox) Vaccine Tests
In this safety study, healthy children received either one or two does of chickenpox vaccine. No placebo.
“Subjects with a negative history of varicella were randomized to receive either one or two injections of the vaccine given 3 months apart.”
Safety and immunogenicity of one vs. two injections of Oka/Merck varicella vaccine in healthy children. A L Ngai, et al., Pediatr Infect Dis J. 1996 Jan;15(1):49-54. doi: 10.1097/00006454-199601000-00011.
Gardasil Vaccine Tests
The most recent vaccine to come to market is Gardasil. With this vaccine, the vaccine maker (Merck) allegedly lied about the placebo. There is a lawsuit pending about this as well as other alleged frauds and negligence.
In their clinical trials:
5,088 children were given Gardasil.
3,470 children were given Amorphous Aluminum Hydroxyphosphate Sulfate (AAHS). This is one of a half dozen distinct forms of aluminum adjuvants.
320 children were given saline. This is only 3.7% of the children who got Gardasil or AAHS.
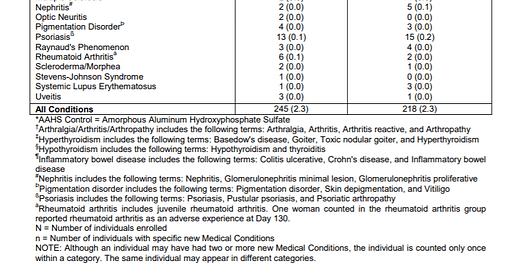
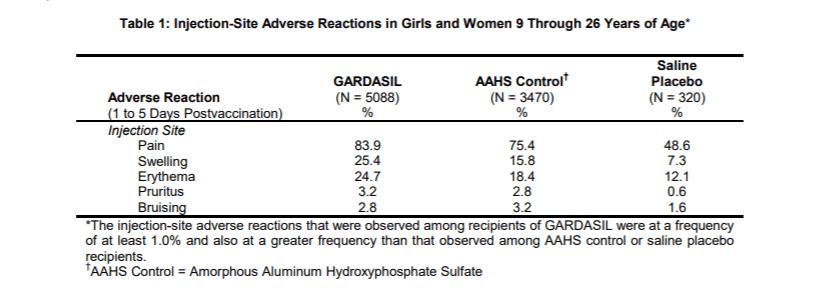
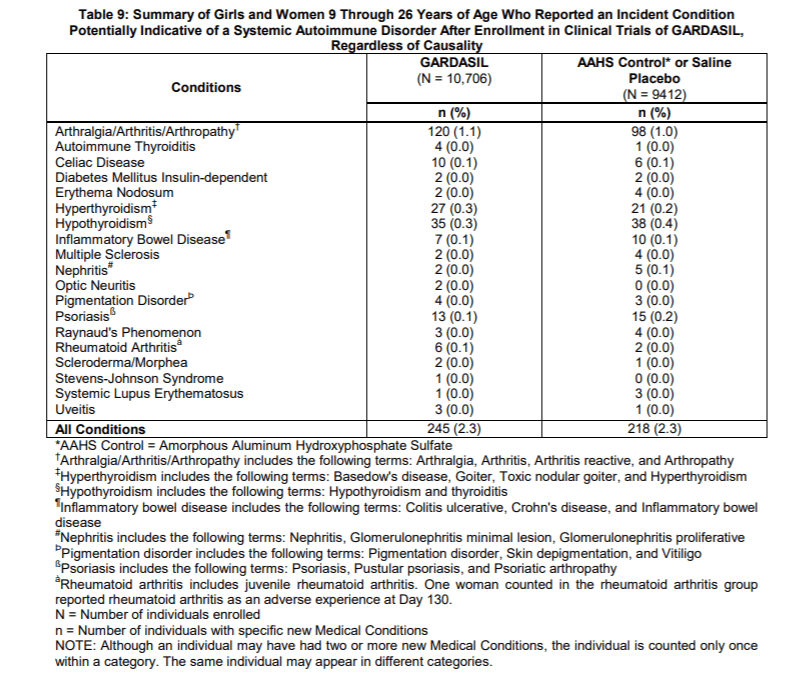
When Merck reported side effects at the injection site like pain, swelling and bruising they broke out all three groups. However, when they reported the more severe side effects like a systemic autoimmune disorders such as arthritis, celiac disease, inflammatory bowel disease, hypothyroidism and psoriasis, they added the children who got saline to those who got aluminum (AAHS). Not surprisingly, 2.3% of both groups reported systemic autoimmune disorders because both groups received toxic substances.
Merck described its chemical Amorphous Aluminum Hydroxyphosphate Sulfate as “a visually indistinguishable aluminum-containing placebo.”
Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions, FUTURE II Study Group. N Engl J Med. 2007 May 10;356(19):1915-27.
A placebo is defined as a substance or treatment which is designed to have no therapeutic value. Common placebos include inert tablets (like sugar pills) and inert injections (like saline). Does Amorphous Aluminum Hydroxyphosphate Sulfate have no therapeutic value? No. Amorphous Aluminum Hydroxyphosphate Sulfate is used because it does have a therapeutic impact as an adjuvant and has an effect on the immune system.
According to the University of Michigan Medical School:
“Merck Aluminum Adjuvant (AAHS) is a proprietary aluminum hydroxyphosphate sulfate formulation that is both physically and functionally distinct from traditional aluminum phosphate and aluminum hydroxide adjuvants. At a macromolecular level, AAHS is structurally related to aluminum phosphate as it forms an amorphous mesh-like structure.”
There is not a lot of information published on AAHS because it is “proprietary.” We are told it “is structurally related to aluminum phosphate.” Here’s some information published about aluminum phosphate from Agency for Toxic Substances and Disease Registry, U.S. Department of Health & Human Services.
“Following intramuscular administration of aluminum hydroxide or aluminum phosphate vaccine adjuvants in rabbits, increased levels of 26Al were found in the kidney, spleen, liver, heart, lymph nodes, and brain (in decreasing order of aluminum concentration) (Flarend et al. 1997). There is also evidence from animal studies indicating that aluminum administered parenterally accumulates to a small extent in the milk of lactating mothers, and that aluminum crosses the placenta and accumulates in fetal tissue.”
(Cranmer et al. 1986; Yokel and McNamara 1985; Yumoto et al. 2000).



What justification do the editors of these journals that publish this sh!t use to accept these methodologically flawed ‘studies’?
Thank you. I just sent this to someone having a baby in a couple of months.