Does ELIQUIS (apixaban) Cause Memory Issues?
People Over 80 May Be Getting a Double Dose - What Their Doctor May Not Know
Some medications can cause memory loss, confusion, or other side effects similar to dementia symptoms. Dosage matters.
ELIQUIS (apixaban) is a novel oral anticoagulant widely prescribed for preventing blood clots in conditions such as atrial fibrillation and risk of stroke. Amidst its growing popularity, concerns about its side effects, particularly regarding cognitive functions like memory, have emerged. Here, we review what is known, what is observed, and what remains a mystery in the context of ELIQUIS and cognitive health.
Understanding ELIQUIS and Its Side Effects
ELIQUIS operates as a factor Xa inhibitor, a class of medication that prevents blood clot formation, a crucial intervention in conditions predisposing individuals to strokes and other serious complications. While its benefits in preventing clotting are clear, the side effects range from common issues like bleeding and dizziness to more severe, albeit less common, effects such as confusion and memory disturbances.
Dosing in the Elderly: A Critical Consideration
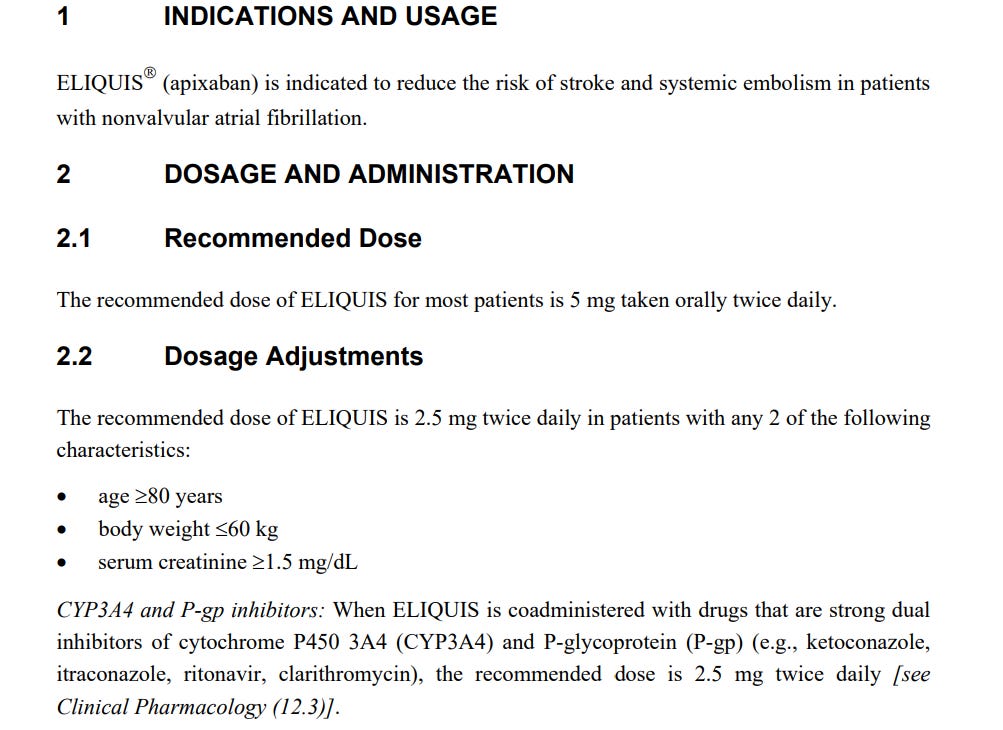
The FDA outlines specific dosing recommendations for ELIQUIS, particularly for the elderly and those with certain health characteristics. For patients over 80 years, weighing less than 60 kg, or with serum creatinine levels above 1.5 mg/dL, the recommended dose is adjusted to 2.5 mg twice daily. This adjustment is vital, as the elderly are more susceptible to the drug's effects due to changes in drug metabolism and increased likelihood of polypharmacy.
Interactions with Other Medications
ELIQUIS's interaction with strong dual inhibitors of cytochrome P450 3A4 (CYP3A4) and P-glycoprotein (P-gp) necessitates a dose adjustment to mitigate increased exposure risks. Medications such as ketoconazole, itraconazole, ritonavir, and clarithromycin, when coadministered with ELIQUIS, require careful monitoring and dosage adjustments to avoid exacerbating side effects, including those affecting cognitive functions.
Memory Issues and ELIQUIS: Piecing Together the Evidence
While memory loss is not even listed as an uncommon side effect, anecdotal evidence and case reports suggest a potential link between ELIQUIS and cognitive impairments. A notable case involved a 60-year-old woman experiencing severe neurological symptoms, including confusion and disorientation, which reversed upon discontinuation of the drug. When she then, unbeknownst to her doctors, re-started the drug, the memory impairment returned. Such cases underscore the importance of vigilance and reporting in detecting rare adverse effects.
The Role of Cerebral Microhemorrhaging
A study highlighting cerebral microhemorrhages in mice administered high doses of ELIQUIS over extended periods offers a possible mechanism for the observed cognitive side effects. These microhemorrhages could theoretically contribute to or exacerbate cognitive symptoms, providing a crucial area for further research, especially concerning long-term high-dose exposure in humans.
What Your Doctor May Not Yet Know
The limitations of pre-FDA approval studies in detecting rare side effects mean that the full spectrum of ELIQUIS's impact on cognitive health may not be fully understood. The intricate balance between anticoagulation benefits and the risk of adverse effects, particularly in the elderly and those with complex medical profiles, necessitates a personalized approach to medication management.
Conclusion
As always, navigate the benefits and risks of ELIQUIS, the dialogue between patients and healthcare providers becomes paramount. ASK YOUR DOCTOR TO CHECK THE PRODUCT INSERT ON DOSING. Awareness of the potential for cognitive side effects, informed by both clinical evidence and patient experiences, is crucial in optimizing care. Further research into the mechanisms underlying these effects will enhance our understanding and guide safer, more effective anticoagulation therapy.
How medications impact cognitive health, advocating for informed discussions and decisions in the journey toward optimal brain health.
FDA PRODUCT INSERT LANGUAGE
ELIQUIS (apixaban) label (fda.gov)
https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdf
“When ELIQUIS is coadministered with drugs that are strong dual inhibitors of cytochrome P450 3A4 (CYP3A4) and P-glycoprotein (P-gp) (e.g., ketoconazole, itraconazole, ritonavir, clarithromycin), the recommended dose is 2.5 mg twice daily”
“The recommended dose of ELIQUIS is 2.5 mg twice daily in patients with any 2 of the following characteristics: age >80 years, body weight <60 kg, serum creatinine >1.5 mg/dL.”
MOUSE STUDY
Oral anticoagulants enhance the cerebral microhemorrhage burden.
Maud Pétrault, Thavarak Ouk, Olivier Pétrault, Michèle Bastide, Régis Bordet, Vincent Bérézowski, 2019. Safety of oral anticoagulants on experimental brain microbleeding and cognition, Neuropharmacology, Volume 155,2019,Pages 162-172, ISSN 0028-3908. https://doi.org/10.1016/j.neuropharm.2019.05.030.
(https://www.sciencedirect.com/science/article/pii/S0028390819301947)
This study aims at determining the ability of clinical-based doses of four oral anticoagulants to transform the onset of a cerebral microhemorrhages (CMH) burden into a symptomatic intracerebral hemorrhage (ICH) in the healthy brain, and precipitate cognitive impairment. Wild-type mice were anticoagulated for 10 days using apixaban, rivaroxaban or dabigatran as direct oral anticoagulants (DOACs), or warfarin as vitamin K-antagonist. Meanwhile, a burden of ∼20 CMHs was induced in the Sylvian territory by intra-carotid injection of cyclodextrin nanoparticles. At bleeding onset, only warfarin provoked deadly hematoma, and dramatically increased mortality (+45%). All the DOACs enhanced CMH burden through a greater number of intermediate-sized microhemorrhages (+80% to +180%). Although silent at onset, both baseline- and anticoagulant-enhanced CMH burdens increased mortality (+11% to +58%) along the following year without statistical difference among groups, and despite cessation of anticoagulation and absence of CMH progression or transformation into ICH. All survivor mice exhibited reduction in visual recognition memory from 9 months. In the healthy brain, DOACs preserve the onset of microhemorrhages from transformation into ICH, and do not precipitate cognitive impairment despite enhancement of CMH burden. High CMH burdens should however be considered for early detection and preventive memory care apart from anticoagulation decisions.
Keywords: Cerebral microbleeds; Cognitive impairment; Oral anticoagulation; Preclinical model; Rodents
RECHALLENGE CASE
Post-marketing reporting of adverse drug events is essential for new medications, as pre-FDA approval studies lack sufficient subject numbers to detect signals for rare events. Prescriptions for the novel oral anticoagulant factor Xa inhibitors (rivaroxaban, apixaban, edoxaban) have equaled or exceeded those for vitamin K antagonists in many clinical settings requiring chronic anticoagulation, and those of injectable heparins for deep vein thrombosis prophylaxis. We report the case of a 60-year-old woman followed for permanent atrial fibrillation who was prescribed apixaban. She rapidly developed worsening neurologic symptoms of imbalance and non-vertiginous dizziness preventing her from walking, headache, diplopia, and confusion/disorientation. Her symptoms began to resolve after stopping the drug, with return to baseline function within 72 h. Unbeknownst to her cardiology care team, the patient chose to re-challenge herself with apixaban at the same dose, producing identical symptoms and again total symptom resolution within 24 h of drug discontinuation. When seen by her physician, her physical examination was unchanged from her pre-treatment baseline. Symptoms did not recur when switched to rivaroxaban therapy.
WEBMD
According to WebMD, Eliquis (apixaban) can cause confusion as a serious side effect. Other serious side effects include very serious bleeding, vision changes, and trouble speaking
WEBMD: Get medical help right away if you have any signs of very serious bleeding, including: vision changes, confusion, trouble speaking, weakness on one side of the body. A very serious allergic reaction to this drug is rare.
https://www.webmd.com/drugs/2/drug-163073/eliquis-oral/details
DISSENTING VIEW - PRIMEMD (THEY DO NOT CONSIDER THE DOSE ISSUE)




"Ask your Doctor"... Given my experience having survived just the past four years, that is a very difficult ask now. Good luck. Seriously! You're going to need it in this low trust environment.
I wonder how the effects of serrapeptase, nattokinase, lumbrokinase and krill oil supplements compare to Eliquis in safety and benefits.