About Those Pfizer Papers and the Denominator FDA Does Not Want You to See
This is not the first time the favorable conclusions of a report ignored the data in a report. The Pfizer documents seem to show they were all just going through the motions, all for show.
When Siri & Glimstad filed their complaint against FDA for failing to produce the documents from Pfizer’s clinical trials on their COVID-19 vaccine, FDA produced a torrent of documents. Among those documents, available via Public Health and Medical Professionals for Transparency, one document stands out. I’ll summarize here some of the report but be sure to get your own copy here. The file you want is “5.3.6 postmarketing experience.pdf”, and the document is entitled “5.3.6 CUMULATIVE ANALYSIS OF POST-AUTHORIZATION ADVERSE EVENT REPORTS OF PF-07302048 (BNT162B2) RECEIVED THROUGH 28-FEB-2021”.
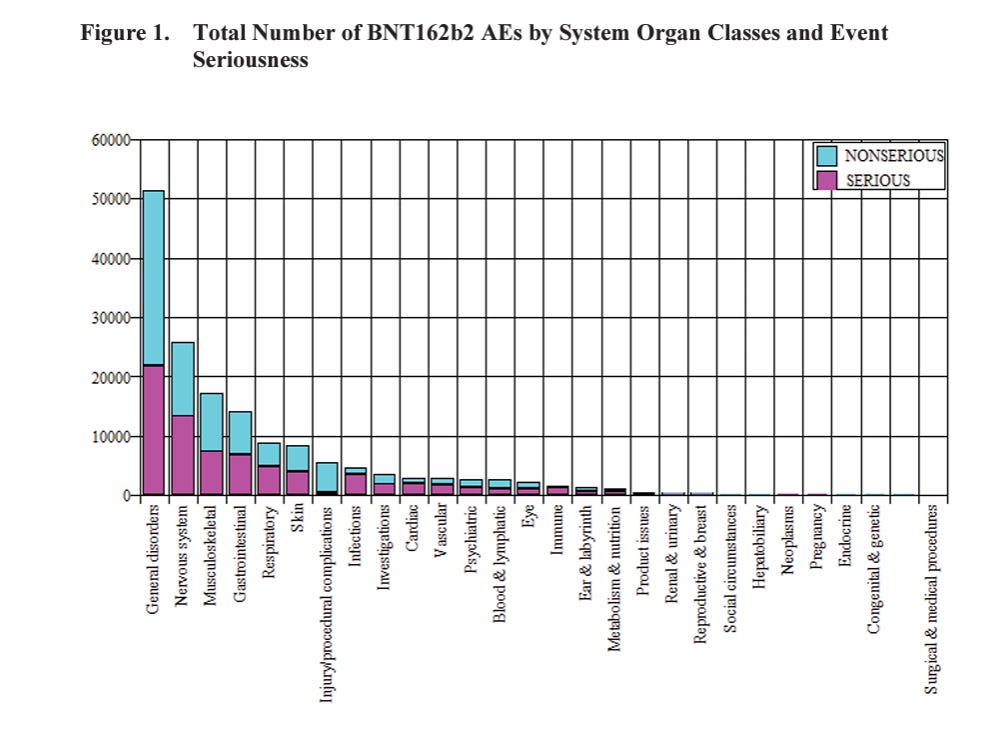
Take a look at Figure 1, which summarizes the 42,086 case reports containing 158,893 events.
I see over 20,000 SERIOUS general disorders, over 10,000 SERIOUS nervous system events, over 5,000 musculoskeletal and gastrointestinal SERIOUS events each.
Pfizer’s Table 2 lists >93,000 events that occurred in ≥2% of events.
Looking at the Figure 1 and the text (serious and non-serious):
Nervous system disorders: 25,957.
Musculoskeletal and connective tissue disorders:17,283.
Gastrointestinal disorders:14,096.
There are more categories, you can see them in Figure 1.
In the meantime:
Remember that Pfizer and Moderna promised complete transparency.
Remember that FDA had access to Pfizer’s numbers.
Remember that these data were not peer-reviewed, and vaccine safety studies data for EUA and FDA approval are not, as a matter of practice, subjected to blinded peer-review. Why not?
What Does This Say About Risk?
Unfortunately, we cannot know. The number of doses given to the date the report was generated was redacted, preventing any computation of rates and risks.
John Campbell points out that Janet Woodcock of the FDA reported, reporting the approval of the EUA on August 21, 2021, said the following:

Knowing she had access to these data, John wonders how could she say that at that time?
John is a pro-vaccine health expert (retired nurse, I presume) in the UK who is now calling out US’s Acting FDA Commissioner. He goes on to call out Peter Marks, director of FDA’s Center for Biologics Evaluation and Research, for saying the following in a press release:
“Our scientific and medical experts conducted an incredibly thorough and thoughtful evaluation of this vaccine. We evaluated scientific data and information included in hundreds of thousands of pages, conducted our own analyses of Comirnaty’s safety and effectiveness, and performed a detailed assessment of the manufacturing processes, including inspections of the manufacturing facilities… (w)e have not lost sight that the COVID-19 public health crisis continues in the U.S. and that the public is counting on safe and effective vaccines. The public and medical community (sic) can be confident that although we approved this vaccine expeditiously, it was fully in keeping with our existing high standards for vaccines in the U.S."
Good for you, John, for calling them out, as we all should. In fact, by redacting the denominator, the FDA may be in contempt of court. They certainly have contempt for public awareness of the risks associated with the Pfizer vaccine.
FDA needs to publish the redacted denominator so we know the rates.
Here you can watch John go step-by-step through the report and conclude that FDA has “destroyed” public trust in the process.





That redaction certainly jumped off that page at me when I browsed those pages, though you are the first analyst who I've seen point it out. Thank you.
The Peter Marks remarks about their "thorough evaluation" refers to the Comirnaty version of the Pfizer vax. Which is not the Pfizer vax that everybody around the world received. They gave regular approval (not just an EUA) to the Comirnaty version. So they did all their lab inspections, and manufacturing checks of Comirnaty, gave it the seal of approval of a regular approval, all at the same time that they were vaxxing with the version that never had a "thorough evaluation".
You can be sure that Comirnaty version that they did test, was minus the additional undisclosed nanocrap.