Why A Cancer Vaccine is Not a Vaccine
It's not Prevention. Formulation for safety matters most. They are immunotherapies.
The recent announcement of the Stargate initiative—a $500 billion project led by Oracle, OpenAI, and SoftBank—has ignited global interest. Central to this effort is the development of personalized cancer vaccines, a technology poised to transform medicine. However, the term "vaccine" here is misleading. Cancer vaccines, or better yet, cancer immunotherapies, differ fundamentally from traditional vaccines in purpose, design, and implementation.
This distinction matters because the success of cancer immunotherapies will hinge on their formulation. By prioritizing safety, precision, and transparency, these vaccines could revolutionize cancer treatment and set a new standard for all immunotherapies. Conversely, a failure to focus on formulation could replicate the shortcomings of traditional vaccines, undermining public trust and limiting their potential impact.
While this moment is represented as a pivotal opportunity, cancer immunotherapies could either validate the promise of precision medicine or serve as a cautionary tale about the consequences of neglecting safety.
The Misnomer of "Cancer Vaccine"
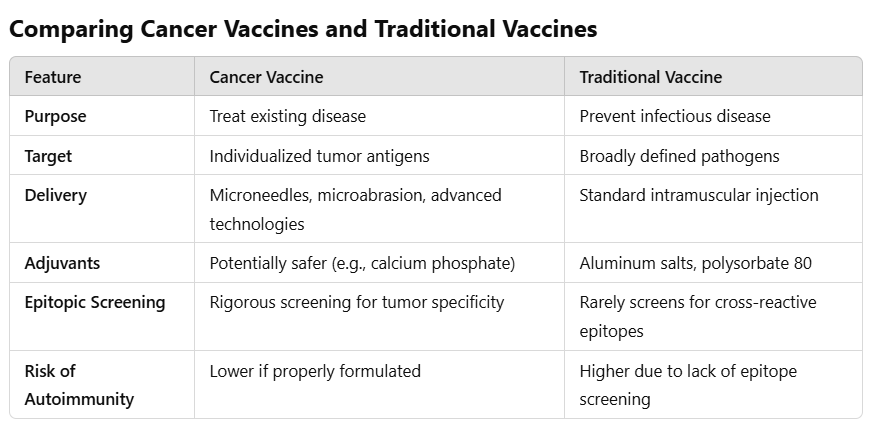
Traditional vaccines aim to prevent infectious diseases like measles or polio by training the immune system to recognize and fight specific pathogens. Cancer vaccines, by contrast, are therapeutic—they treat existing diseases rather than prevent them.
Key differences between these two paradigms include:
Personalization: Cancer immunotherapies are tailored to the unique genetic mutations of an individual’s tumor.
Dynamic Adaptation: Unlike traditional vaccines, cancer immunotherapies may need to evolve alongside the tumor to remain effective.
Non-Universal Application: Cancer immunotherapies target a single patient’s tumor, not an entire population.
These differences necessitate a distinct approach to their design, with far greater emphasis on precision and safety.
Why Formulation Matters
The formulation of cancer immunotherapies will determine their success. Unlike traditional vaccines, which often follow a "one-size-fits-all" approach, cancer immunotherapies demand careful consideration of three critical elements:
Epitopic Specificity:
Cancer immunotherapies must target tumor-specific antigens (TSAs)—mutations or proteins unique to cancer cells. Screening for these antigens is crucial to avoid pathogenic priming, where the immune system learns to mistakenly attack healthy tissues.
Traditional vaccines do not undergo this level of scrutiny. For example, molecular mimicry has been implicated in autoimmune reactions like Guillain-Barré syndrome and narcolepsy following vaccination.
Adjuvants and Additives:
Cancer immunotherapies should prioritize safer adjuvants, such as calcium phosphate, which enhance immune responses without triggering systemic inflammation.
Traditional vaccines often rely on aluminum salts or polysorbate 80—ingredients associated with inflammatory responses and long-term health concerns.
Delivery Mechanisms:
Cutting-edge methods like microneedles or microabrasion could enable localized immune activation, reducing systemic side effects.
Traditional intramuscular injections disperse their contents widely, which may amplify the risk of adverse reactions.
Cancer immunotherapies could set a precedent for safer and more effective immunotherapies by focusing on these knowledge-based principles.
Learning from the Past
The history of traditional vaccines provides valuable lessons. While vaccines may have their place, their formulation has not always prioritized safety. For instance:
The lack of epitope screening has led to documented autoimmune risks.
The inclusion of controversial excipients has fueled public mistrust, even among vaccine supporters.
These shortcomings underscore the need for rigorous formulation standards in cancer immunotherapies development. This new generation of vaccines must succeed on a patient-by-patient basis, demanding a higher level of precision than traditional vaccines.
A Pivotal Opportunity
For cancer immunotherapies to fulfill their promise, stakeholders must focus on safety and personalization. This includes:
Rigorous Epitope Screening: Ensuring that included antigens do not mimic normal human proteins.
Avoiding Harmful Additives: Replacing risky adjuvants and excipients with safer alternatives.
Transparent Data Sharing: Publishing safety and efficacy data, including long-term outcomes.
Setting a New Standard: Cancer immunotherapies could redefine what it means to create safe and effective immunotherapies.
If these principles are followed, cancer immunotherapies could usher in a new era of precision medicine. However, if safety is neglected, this pivotal moment could devolve into another chapter of public skepticism and missed potential.
Conclusion
The development of cancer immunotherapies represents a defining moment for immunotherapy. By prioritizing safety and learning from the shortcomings of traditional vaccines, these treatments could not only transform cancer care but also establish a new gold standard for all vaccines. This is an opportunity to demonstrate that innovation and safety can go hand in hand—an achievement that could redefine public trust in medical science for generations to come.
The success of cancer immunotherapies will depend on their formulation. If safety and efficacy are prioritized, especially epitope screening and avoiding aluminum, this initiative could be remembered as medicine turning a corner toward a safer, more personalized future. However, if old mistakes are repeated, it may only deepen the challenges facing medicine today.




I sure wish they'd spend more time and money figuring out why people get cancer. But I guess that is not profitable.
If I get cancer I will pass on even an individualized cancer vaccine. I'll develop my own personalized plan which may include natural herbal and food remedies, Tippens protocol, rife machine, essaic tea, CDS and a host of other options.