Urgent Concerns Regarding At-Home Administration Approval of FluMist Nasal Vaccine. FDA Must Now Reverse Course.
FluMist is a clear risk to public health, and FDA knows it.
This is a professional analysis of unmitigated risk of shedding that is expected to occur in persons vaccinated. Vaccine mandates are unwise and should be ended. All vaccines that do not prevent transmission should be given blackbox warnings.Vaccines that increase the risk of infections of any kind should be banned. Psychotropic drugs that come with a risk of psychosis must be banned.
The recent FDA decision to approve the at-home administration of FluMist, the live-attenuated nasal influenza vaccine, is a real concern. This particular approval opens up a range of potential public health risks that may disproportionately affect the most vulnerable members of our communities. Given the complexities of FluMist’s live viral formulation and the ease of transmission through respiratory droplets, careful examination is crucial to ensure that broadening access does not inadvertently place millions of people at risk.
FluMist recipients are advised to avoid close contact with immunocompromised individuals for at least 21 days due to viral shedding. Although this precaution targets individuals with immunocompromised household members, the risk extends far beyond the home. Millions of Americans, including those with cancer, HIV/AIDS, autoimmune disorders, and other chronic conditions, are immunocompromised and thus highly susceptible to even attenuated viral strains. Many of these individuals share workplaces, schools, and public spaces with others who may have recently received FluMist.
Without consistent oversight, at-home administration will expose immunocompromised populations to live viral particles in everyday environments. In classrooms, workplaces, and other shared spaces, immunocompromised individuals face involuntary and unknowing exposure, creating a risk of infection for those whom the vaccine is meant to protect.
Retail stores and pharmacies, like Walmart, have also been authorized to offer FluMist, raising critical questions about safety protocols in these high-traffic areas. Without stringent policies, viral particles from recent recipients could remain on surfaces and items throughout the store, unknowingly exposing customers with compromised immune systems. The viral particles will go wherever the recently vaccinated go.
No control measures are conceivable to enforce universally. Casual exposure to surfaces, shared areas, or items handled by recently vaccinated individuals may be all it takes for an immunocompromised person to encounter these viral particles, endangering their health.
Concerns also arise about FluMist’s formulation. Each 0.5 ml dose contains 10 million and 100 million live viral particles. While developed in specific pathogen-free chick kidney cells and eggs, this manufacturing process does not guarantee complete freedom from all contaminants. Thus, the risk of avian viral remnants, though minimized, is not entirely eliminated. In an unsupervised, at-home setting, this formulation complexity presents an additional, unmanaged variable for which adverse reactions may be less readily observed or controlled.
Another critical concern is the neurological risk associated with the nasal administration route. FluMist’s delivery directly into the nasal passages brings it into close contact with the olfactory nerves, which are separated from the brain only by the cribriform plate. Research has shown that certain viruses can reach the brain through this olfactory pathway, which could lead to encephalitis and other neurological issues, particularly if administered improperly. Without trained administration, the likelihood of improper application and exposure of sensitive neural structures increases. This risk warrants careful consideration when scaling up FluMist’s usage across diverse home environments with varying levels of understanding about application methods.
Furthermore, while the aim of generating herd immunity by targeting healthy individuals aged 5 to 49 with FluMist is intended to protect high-risk groups, it will instead place these populations in greater danger. High-risk groups, including young children, the elderly, and those with compromised immune systems, are more likely to encounter vaccinated individuals within their communities, potentially exposing them to the attenuated virus through viral shedding. This unintended outcome may counteract the public health objectives of FluMist administration, inadvertently amplifying rather than mitigating risk for these populations.
In light of these critical issues, the FDA must reverse course for approval, beginning with at-home administration of FluMist, and to anyone who cannot guarantee they will not - knowingly or unknowingly - anyone who is immunocompromised. While enforcing rigorous safety protocols for retail stores and pharmacies offering FluMist, such as designated vaccination zones, mandatory hand hygiene policies, and enhanced sanitation requirements, could help minimize unintended viral transmission in public spaces, there are no conceivably effective safeguards to protect vulnerable populations in the general population. A policy restricting at-home administration in households with known immunocompromised individuals, and specific guidelines to reduce exposure in workplaces, schools, and other shared environments, will not be effective - and would be unenforceable. Further research is also essential to fully assess the neurological risks associated with FluMist’s intranasal delivery.
Protecting our most vulnerable community members requires a cautious, well-regulated approach. The FDA must be directed to ensure that the health of all Americans—especially those most susceptible to unintended consequences—is safeguarded. All Americans are urged to take these concerns seriously and to expect the FDA to implement measures that reflect a commitment to safety.




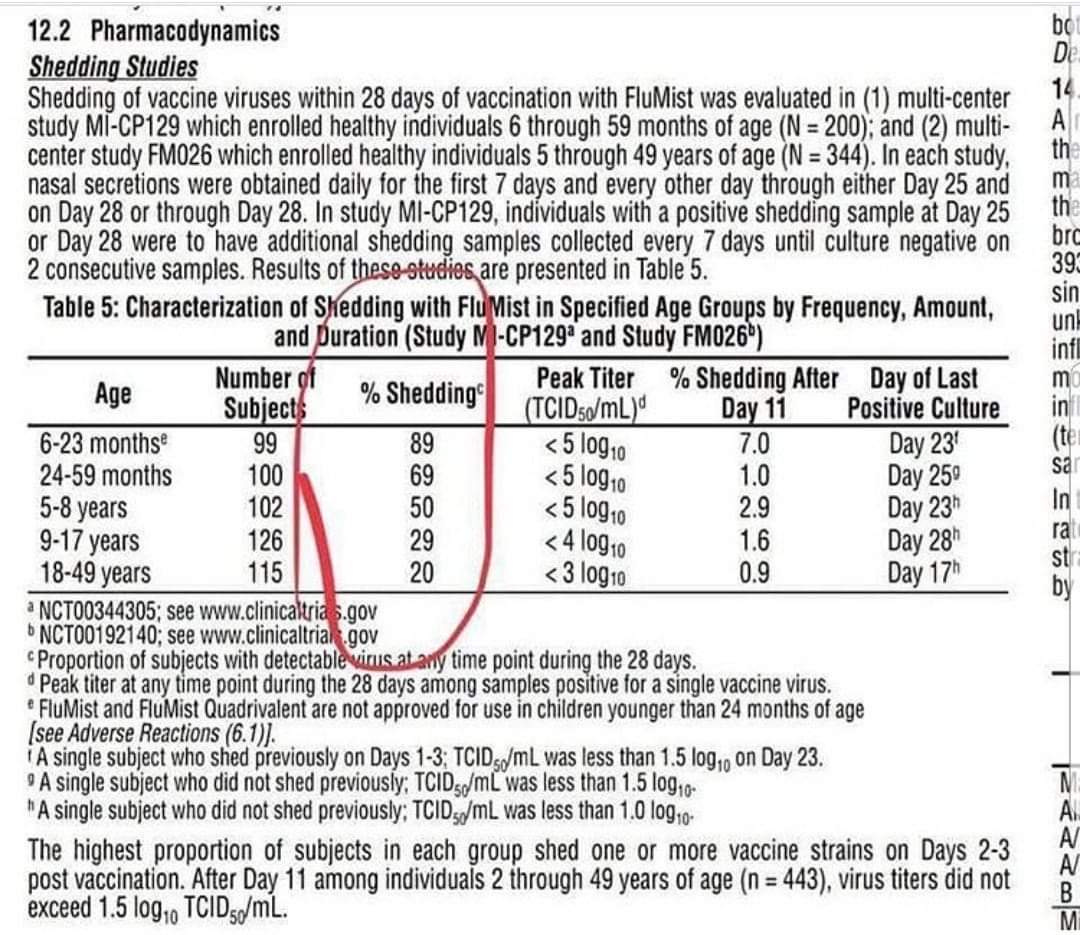
All products purported to contain live viruses are extremely dangerous, as there is no way to control nor determine their potency after they get distributed into the market. This product is going to make a lot of people sick, even those who are secondarily exposed through the shedding process they admit does happen.
The FDA has a 30% failure rate for drug safety and efficacy. I say fire the lot as part of the government downsizing effort. Start house cleaning with Letter agencies. Resume American Manufacturing Independence and Dominance or Expect To Die Communist.