Survivorship Bias: Avoiding Skewed Understanding of Long-Haul COVID
In sculpture, artists use space as much as form to convey a message. Nature leaves spaces, too and we've missed a big realization about Long-Haul COVID
Peer into the immunological literature on COVID, if you dare. Starting with Pathogenic Priming, it’s now massive, with nearly 46,000 published manuscripts in Pubmed. And a consistent theme has been there from the start: COVID-19 involves some form of attack on immune proteins, and thereby, impairs the immune system. It does so, it seems, in part due to autoimmunity triggered by SARS-CoV-2 viral proteins.
Long-Haul COVID (LHC) was mostly a mystery until Bruce Patterson, MD revealed in mid-to-late 2020 that sufferers had a dearth of CD8+ cells. CD8-positive T-cells are important members of MHC class I-restricted T-cells and are mediators of adaptive immunity. Bruce and his colleagues found that sufferers also failed to form germinal centers, which are the dōjō of evolutionary competition between B-cells that are competed against each other for survival based on the exactness of the fit of their antibodies to the antigen of the day - in this case, whichever SARS-CoV-2 protein a population of B-cells has been instructed to attempt to develop antibodies against.
Without sufficient CD8+ T-cells, the immune system cannot efficiently clear the body of SARS-CoV-2 infected cells.
In December, we learned that the anti-inflammatory responses, specifically cytokine inhibition and tissue repair rates, also positively correlate with T-cell number and are significantly suppressed in non-survivors (Zhang et al., 2021).
We also learned that in healthy survivors without LHC, we not only see a “robust” CD*+ T-cell and corresponding B-cells, we also see a healthy Treg response in hospitalized patients who have good clinical outcomes (Caldrer et al, 2021).
It’s more than a year since I interviewed Dr. Patterson on Jan 31, 2020 about this, and the literature has exploded. A broad overview of the literature on long-haul COVID now seems to include the fact that sufferers are not only unable to create trained, adaptive T-cells well-suited to the task of recalling which cells to kill based on their surface antigens, but also that LHC patients have a dearth of naïve B- and T-cells as well.
In this study, the T- and B-cell populations of people with SARS-CoV-2 were compared to people who had infections from other human coronaviruses than SARS-CoV-2 (these included HCoV-NL63, O229E, OC43 or HKU1).
The researchers reported:
“Patients with LC had highly activated innate immune cells, lacked naive T and B cells and showed elevated expression of type I IFN (IFN-β) and type III IFN (IFN-λ1) that remained persistently high at 8 months after infection.”
Ok, big picture time.
In people with mild to moderate COVID-19 who do not progress to LHC, the cytotoxic CD8+ T-cells are active, but don’t go crazy.
In people who progress to severe COVID-19, cytotoxic CD8+ T-cells go crazy (unregulated), implying problems w/Tregs.
In survivors with LHC, CD8+ T-cells are just plain missing, along with naïve T- and B-cells, including T-regs, a subset of CD4+ cells.
This would seem to imply that stimulation of T-cell production against SARS-CoV-2 is best done in the absence of past cold virus infection (how recent, we don’t know).
Baseline Differences
Most of the studies in the literature are considering these differences as different effects of the virus on the immune system response. But there is an equally compelling literature on observations that people going into COVID-19 may be pre-determined to have particular clinical courses based on baseline differences in their immune systems.
For example, 78% of people who progressed to severe COVID-19 had autoantibodies to something else in their body prior to infection, whereas only about 8% of patients with mild COVID-19 already had autoimmunity.
In this complex setting then, difference such as “missing T-cells” must be checked against pre-infection immunological status.
Patients and doctors alike need an immunological dashboard to know where they stand. And, as I’ve said before, in a world with viruses like SARS-CoV-2, all physicians should take a course in immunology.
Survivorship Bias?
One of the main confounders in the entire field of study is the fact that people with past coronavirus infection survive, which is a good thing, of course, as do people with mild-to-moderate COVID and with LHC. In the Nature Immunology study, the clinical severity and outcomes in the endemic HCoV group were not broken down into mild, moderate, severe, or “died”. It’s just “the HCoV group”. So to compare T-cells (or anything else) between people with LHC and the HCoV group is at risk for an unseen survivorship bias. More on survivorship bias in a minute…
Perhaps it’s the SARS-CoV-2 infected patients who died that had higher T-cell counts, leaving behind people who tend to not produce as many T-cells. Like the elderly.
I’m not saying this explains all of the variation in outcome; only a noob would find a confounder and say that this means that other factors cannot be responsible (such as the noob that managed to get my study with Paul Thomas retracted based on his speculation over a potential confounder, and the journal bought it, hook, line and sinker).
I’m saying that if we are to profoundly understand the role of T-cells in affecting the outcomes we have to remember that those who died took away from the population of survivors, leaving space.
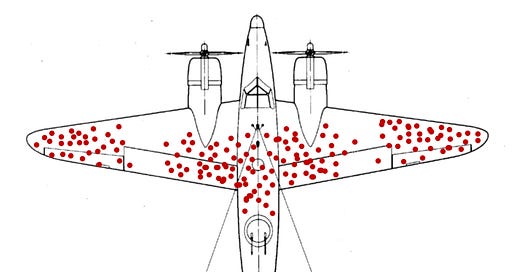
What is Survivorship Bias?
In World War II, US airplanes returning to bases after flying missions showed a discernable pattern where they had been shot up. The wings and the tails seemed to receive more hits than the rest of the plane, and so the US military wanted to increase the armor in the areas most hit by bullets.
That’s when Abraham Wald, a refugee immigrant mathematician in the US who had escaped from Nazi-occupied Austria, realized the bigger picture.
Martin Grandjean (vector), McGeddon (picture), Cameron Moll (concept), CC BY-SA 4.0, via Wikimedia Commons
Wald pointed out that the areas of the plane where the bullet holes were not found were actually evidenced to be more vulnerable, and that the bullet holes tended to be missing from those areas of the plane because when planes were hit in those areas, the planes did not make it back. When the US military increased armor to the places where the planes coming back had not been hit, the rates of losses plummeted. There are plenty of articles on this across the web.
Survivorship Bias and T-cells
In this logical breakdown “T-cell type X” is not meant to imply an existing T-cell, but rather any T-cell, including those that are missing (as in lymphopenia).
If people who do not survive have T-cell type X, then people who do survive have T-cell type Not X.
If people who survive without LHC have T-cell type Y, then people who survive with LHC have T-cell type Not Y.
Therefore, it is desirable to have T-cells Not X and also T-cells Y.
The people who survive COVID-19 can only show us by comparison to the people who do not survive COVID-19 which T-cell responses are healthy. A comparison of severe vs. mild COVID-19 or hospitalized vs non-hospitalized COVID-19 patients would be effective.
The people who survive and develop LHC can only show us by comparison to people who survive and do not develop LHC the types of T-cells that are desirable.
Direct comparison on surviving patients with LHC and patients without LHC has already been done, as I noted, by Bruce Patterson and colleagues, who originally pointed out in 2020, reveal a lack of CD8+ T-cells in LHC, and as logic dictates, a lack of over-reactive cytotoxic T-cells in survivors in general.
All of this analysis includes immune reactions to the SAR-CoV-2 virus, not the spike-only vaccines. Unfortunately, most of the comparisons of cell-mediated immunity from vaccines vs. from infection involve measurements made only on the spike protein. There have been notable exceptions, and certainly clinical experience and prospective studies have shown that immunity from infection is longer-lasting (13 months and counting from infection vs. 3-4 months from vaccination).
It now seems likely that long-term immunity against SARS-CoV-2 involves antibodies and immunology memory for those antibodies in B- and T-cells trained on the essential proofreading endonuclease, not the spike protein.
The take-home is that when reading about differences in characteristics about patients with different outcomes, one must always ask (a) were their baseline difference that carried through the study from start to finish, and (b) is there a survivorship bias and should we, therefore, focus on the antithesis of our thesis on causality?
My assessment in all of this is that patients with lymphopenia will be found to have poor hematopoietic stem cell differentiation overall. This takes place in bone marrow.
What factors harm healthy immune cell differentiation?
Age
Micronutrient deficiency
Repeated radiation of the long bones
Aluminum accumulation in the bones
Environmental toxins
Hodgkin's disease
Lupus
Infectious diseases like tuberculosis, AIDS and viral hepatitis
Parasite infections
Now we see why people with comorbidities do not fare well with COVID-19. Now we see why being elderly is the primary risk factor for serious COVID-19.
Ironically, these same people may have more trouble with COVID-19 vaccines, so we have to address both the risk factors for severe COVID-19 and for vaccine adverse events.
This is a complex way of saying that we should protect those vulnerable to both risks, and whether it’s the baseline, SARS-CoV-2 protein-mediated harm, or both. And that starts with immune health.




Wonderful easy to understand article. I also don’t think we can leave out the massive amounts of biologics that have been prescribed over the past two decades.
Fascinating, thanks!
Hypervitaminosis A or D also affect immune cell differentiation, as do topical creams containing retinol or retinoic acid.