Statement of Concern over Moderna's mRNA-1083 Bivalent COVID19/Influenza Vaccine Trial
Combined Phase1/Phase 2, Counting Window Bias Placebo Results Not Reported, General Underreporting of Available Results Impair Assessment of Safety and Efficacy
OUTLETS: PLEASE REPUBLISH WITHOUT CHANGE. - JLW
Moderna’s most recent mRNA-1083 trial, a Phase 3 clinical investigation, aims to evaluate the immunogenicity, safety, and reactogenicity of Moderna's investigational combination vaccine against influenza and COVID-19. This trial involves two cohorts: adults 65 and older and adults aged 50 to 64. Each cohort is designed to compare the mRNA-1083 vaccine with co-administered licensed influenza and COVID-19 vaccines. The primary goal is to determine if mRNA-1083 can elicit a stronger immune response while maintaining a safety profile comparable to established vaccines. All of the comments here are based on the limited information provided by Moderna and ClinicalTrials.gov to the public.
The trial design and subsequent communications reveal several important concerns. These concerns are not new to our readers; the modus operandi has been to use study designs that inflate efficacy and hide significant adverse events. In this article, a comparison of the study as proposed and described at ClinicalTrials.gov and as described by Moderna in their press release shows cause for concern for physicians, patients, and shareholders alike.
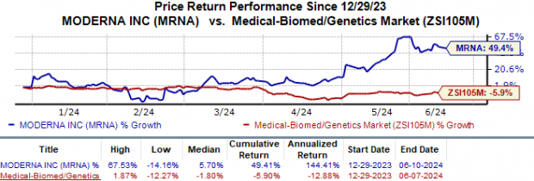
MODERNA STOCKS ARE UP
According to MSN, "Moderna's shares have surged 49.4% against the industry's 5.9% fall"
THE MISSING PHASE 2 TRIAL: LOSS OF SEQUENTIAL RISK ASSESSMENT
The first issue is the missing Phase 2 trial. In an earlier press release on this vaccine, Moderna had reported on results from a “Phase 1/2 Trial”. There appears to be a consistent pattern to avoid doing full-on Phase 2 trials; in the first COVID-19 vaccine, Phases 2 and 3 were “combined”, which has no meaning in translational research. However, in Phase 2, adverse events are first detected, so follow-up on adverse events can be done in Phase 3. In this new combined COVID/flu vaccine trial, Phase 2 was combined with Phase 1. The specific details on the effects of this on finding adverse events are not available, and they never will be because Phase 1 and Phase 2 results are entwined.
COUNTING WINDOW BIAS
An extremely critical issue is counting window bias. Counting window bias, also known as the Lyons-Weiler/Fenton/Neal effect, occurs when the period during which outcomes are measured does not accurately capture the true incidence or timing of those outcomes. This bias will significantly distort both the reported rates of adverse events and those of COVID-19 cases, leading to misleading conclusions about a vaccine's safety and efficacy. Lyons-Weiler first reported this effect in Children’s Health Defense The Defender, and Fenton and Neal later also reported it via their Substack.
In the context of the mRNA-1083 trial, if the window for counting COVID-19 cases or adverse events starts too late, early occurrences directly related to the vaccination might be missed. For instance, if adverse events are only recorded several days after vaccination, immediate reactions within the first few days will be entirely missed. This design flaw leads to underestimating the vaccine's reactogenicity, providing an incomplete safety profile. Immediate adverse reactions, such as injection site pain, fever, or allergic responses, are critical for understanding the short-term risks associated with vaccination.
Similarly, if the counting window for COVID-19 cases does not begin until a certain period post-vaccination, any cases that occur in the immediate aftermath might not be counted. This could prevent the detection of any increased risk of infection due to disease enhancement or immune suppression caused by the vaccine. Undercounting these early cases can lead to overestimating the vaccine's efficacy in the initial weeks following vaccination.
Counting window bias can also affect the assessment of long-term outcomes. Late-onset adverse events might be missed if the follow-up period is insufficient. This is especially important for vaccines that might have delayed side effects, which could only manifest weeks or months after administration. A follow-up period that is too short fails to provide a comprehensive view of the vaccine’s safety over time.
NO PLACEBO COMPARISON REPORT IN THE PRESS RELEASE. SHOW US THE DATA!
Another persistent major issue is omitting results from a placebo group comparison in the press release.
A placebo group was, however, included in the ClinicalTrials.gov description:
“Experimental: Cohort B1: mRNA-1083 and Placebo Participants of age (sic) 50 to <65 years will receive mRNA-1083 and placebo administered as 2 IM injections of on Day 1. Biological: mRNA-1083 Suspension for injection Biological: Placebo 0.9% sodium chloride suspension for injection”
This omission could mislead stakeholders, potentially inflating the perceived efficacy and safety of mRNA-1083. This concern repeats the NextCOVE study reports (NCT05815498), in which the absence of a placebo control group resulted in underreported adverse events and an overstated sense of vaccine safety. Consistent inclusion of placebo groups is essential to provide a baseline reference and accurately assess a vaccine's true efficacy and risk profile.
The clarity and specificity of comparator groups are another point of concern. The press release explicitly names the licensed vaccines used for comparison: Fluzone HD®, Fluarix®, and Spikevax®. In contrast, the ClinicalTrials.gov description refers to these comparators more generally as "age recommended (sic) quadrivalent influenza vaccine and COVID-19 vaccine." The lack of specific detail can affect the transparency and understanding of the trial. Clear and consistent information about comparator groups, whether any or all were included/excluded, is essential for understanding the context and validity of study findings, an issue also noted in the NextCOVE study, where detailed information about comparators was sometimes lacking.
Detailed outcome measures are crucial for a comprehensive understanding of vaccine performance. The press release focuses on immune response metrics and broad safety profiles, mentioning GMRs and confidence intervals for both cohorts. However, the ClinicalTrials.gov description provides more detailed primary and secondary outcome measures, including specific assays, seroconversion definitions, and tracking of various adverse events. The summary in the press release lacks these detailed metrics, potentially contributing to an overestimation (or overstatement) of efficacy and an underestimation (or understatement) of adverse events. The concern is that the full spectrum of results is not fully communicated in press releases. Similar concerns were raised in the NextCOVE study, where the absence of detailed outcome measures led to an incomplete understanding of the vaccine's true impact.
The potential lack of full reporting of safety data is another significant concern. The press release states that mRNA-1083 showed an acceptable tolerability and safety profile, with most adverse reactions being grade 1 or 2. The ClinicalTrials.gov description, however, outlines detailed measures for solicited and unsolicited adverse reactions, medically attended adverse events, adverse events of special interest, and serious adverse events. The press release's summary might not fully convey the extent and severity of potential adverse events. Comprehensive safety data reporting is essential for evaluating the true risk profile of the vaccine. Inadequate safety data reporting, as seen in the NextCOVE study, led to the severe underestimation of potential risks.
Differential timing of vaccination within the study population can introduce additional bias. If participants receive their vaccinations at different times but the counting window starts uniformly, those vaccinated earlier have a longer period during which adverse events or COVID-19 cases can be recorded compared to those vaccinated later. This discrepancy can create artificial differences in the data, where early vaccinators appear to have higher rates of events simply because they are observed for a longer period.
To address counting window bias, it is essential to ensure that the measurement period accurately reflects the true incidence and timing of outcomes. This can be achieved by starting the counting window immediately after vaccination and maintaining a consistent participant follow-up period. Detailed reporting should include immediate and delayed outcomes to capture the complete spectrum of potential adverse events and efficacy data.
The implications of these discrepancies for public health are profound. Underreporting adverse events and omitting critical details can lead to a false sense of security regarding the vaccine’s safety profile. This misrepresentation can result in widespread use without fully understanding the risks, a concern similarly raised with Moderna’s earlier trials, including their (and others’) initial COVID19 vaccine and the NextCOVE study. Misleading safety profiles can erode public trust and lead to unforeseen public health challenges.
Due to the omission of a placebo group and lack of detailed data, inflated efficacy claims can lead to an overreliance on vaccines as a public health measure, leading individuals and their physicians to miss the opportunity for effective therapies and treatments. This could increase hospitalization rates, deaths, and misallocation of resources. The NextCOVE study also faced criticism for potentially overstating efficacy, leading to similar concerns about the effectiveness of public health strategies.
The exclusion criteria, which omit high-risk populations such as individuals with underlying health conditions or immunocompromised, limit the data available for these vulnerable groups. This exclusion undermines public health strategies aimed at protecting these populations, as seen in the NextCOVE study, where similar exclusions led to gaps in understanding the vaccine's performance in high-risk individuals.
Discrepancies and lack of transparency can erode public trust in vaccination campaigns and public health authorities, fueling vaccine hesitancy. The NextCOVE study's transparency and detailed reporting issues also led to concerns about public trust, highlighting the importance of clear and comprehensive communication in maintaining public confidence.
Misleading or incomplete data can impact public health policies, leading to ineffective vaccination strategies and resource allocation. Inadequate reporting and transparency was seen in both the prior NextCOVE trial as well as the new mRNA-1083 trial. This can complicate policy decisions and hinder effective public health responses.
INCLUSION/EXCLUSION IMPARTS TRANSLATIONAL RESEARCH BIAS
The inclusion and exclusion criteria for the mRNA-1083 trial could introduce several biases that may impact the generalizability and interpretation of the study results. By including only healthy adults and excluding those with acute illnesses, chronic conditions, or recent use of immunosuppressants, the study population may not fully represent the broader, more diverse population that would receive the vaccine in real-world settings. This could lead to an overestimation of the vaccine's safety and efficacy, as healthier individuals are likely to have stronger immune responses and fewer adverse events compared to those with underlying health conditions. Additionally, excluding participants who have received recent vaccinations or tested positive for influenza or COVID-19 could further skew the results, as it does not account for potential interactions between the combination vaccine and other vaccines or prior infections. These criteria might result in a study cohort that is not reflective of high-risk groups, such as the elderly or those with comorbidities, thereby limiting the applicability of the findings to these crucial populations who are often considered to be most in need of protection from serious illness from respiratory viral infections.
The identified discrepancies and concerns in the mRNA-1083 trial's design and reporting raise significant questions about its validity, reliability, and applicability to real-world settings. The issues identified, such as the omission of placebo groups, lack of detailed comparator information, incomplete safety data reporting, and exclusion of high-risk populations, are consistent with concerns previously noted in the NextCOVE study (NCT05815498). These problems can lead to misrepresenting vaccine safety, efficacy overestimation, public distrust, and policy challenges.
To ensure reliable vaccine safety and efficacy assessment, physicians, patients, and shareholders should expect full reports on future trials to incorporate rigorous, transparent, and comprehensive research practices. The use of proper study designs, including properly counting cases, hospitalizations, and deaths following the administration of the first dose is essential to curtail the massive bias induced by restricting counting to events that occur weeks after the second dose. Detailed and accurate reports, including the result of comparisons to placebo controls and comprehensive safety data in press releases, are essential for informed public health decisions. Addressing these issues is vital for acquiring and deserving public trust.
RELATED
CNN: Moderna says Covid-flu combination vaccine shows positive results in late-stage trial
'Bad Optics' Or Something More? Moderna Executives' Stock Sales Raise Concerns (michiganpublic.org)








“… while maintaining a safety profile comparable to established vaccines.”
.
We Didn’t Need To Find The Cure For Cancer.
We Found The Cause.
.