Rushed Science, Reckless Oversight: The Unseen Risks of RNA Injectables
A new study by Ulrike Kämmerer, Verena Schulz, and Klaus Steger replicates, validate and extends previous studies.
The rush to bring vaccines to market in response to the spread of SARS-CoV-2 across the globe left critical safety measures and scientific rigor as an afterthought. A new peer-reviewed study by researchers from the University Hospital of Würzburg, Department of Obstetrics and Gynecology, Würzburg, and the Justus Liebig University, Giessen, Germany, published in Science, Public Health Policy and the Law has reported troubling findings about the BNT162b2 injectable, raising serious questions about regulatory oversight, manufacturing processes, and the long-term implications of these groundbreaking RNA-based interventions.
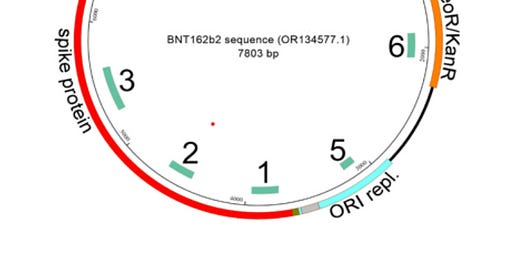
The study’s findings are disconcerting. Researchers discovered significant levels of residual plasmid DNA in all four BNT162b2 lots they analyzed—up to five times the regulatory limit. Even more alarming is the presence of an SV40 promoter/enhancer sequence, a genetic element associated with nuclear transport in mammalian cells. The implications are profound: these elements may facilitate genomic integration, a possibility that raises the specter of unintended genetic alterations in human cells.
The undisclosed inclusion of the SV40 sequence—a choice that appears neither necessary for the production of spike at the ribozyme, nor necessary for RNA injectable production process—raises troubling questions about the manufacturing decisions behind these biologics. Why include a promoter known for enabling nuclear entry, mainly when it is not essential to the mRNA platform? Depopulation concerns notwithstanding, these findings suggest, at best, a lack of careful deliberation and, at worst, a reckless disregard for potential risks.
Adding to the concerns is the discovery that transfection of human HEK293 cells with these injectables resulted in prolonged spike protein production, with secretion facilitated by extracellular vesicles. The systemic distribution of spike proteins through this mechanism raises new uncertainties about the biological impact of these injections on human recipients. While the manufacturers initially claimed localized and short-lived expression, this study demonstrates otherwise.
The findings in this study confirm and extend results from previously published research on the residual DNA content and its potential implications in RNA-based COVID-19 injectables. Specifically:
Residual DNA in Pfizer-BioNTech Lots:
The study corroborates earlier findings by McKernan et al., who identified residual plasmid DNA in Pfizer-BioNTech lots. This included DNA concentrations exceeding regulatory limits and fragments of plasmid DNA used in the manufacturing process. These similarities validate the robustness of the current study’s findings regarding DNA contamination.Presence of SV40 Promoter/Enhancer:
The detection of the SV40 promoter/enhancer sequence aligns with prior reports by McKernan and others. The 72-base pair spanning element of the SV40 promoter/enhancer, found in two copies, is known for its ability to facilitate nuclear transport of plasmid DNA, and its presence in the injectable lots raises significant safety concerns about unintended genomic integration. This study builds on that earlier work by confirming the presence of SV40 sequences in additional lots and their potential for transfection in human cells.Spike Protein Persistence:
Previous studies have reported the extended presence of injectable-induced spike proteins in the bloodstream of recipients, sometimes weeks after vaccination. The current study’s demonstration of prolonged spike protein production in cell cultures, along with evidence of extracellular vesicle-mediated secretion, provides mechanistic insights into these clinical observations. It suggests that the persistence may not be limited to isolated cases but could be a feature of the biologic’s design and delivery system.DNA Packaging in Lipid Nanoparticles:
The study’s findings about DNA being encapsulated in lipid nanoparticles also echo earlier concerns raised by researchers. Reports of inefficient separation of residual DNA during manufacturing are consistent with the results presented here, which showed a marked increase in DNA content after treating the nanoparticles to release their contents.
The study provides a robust and diverse body of evidence to substantiate its findings on the presence of residual DNA, the inclusion of SV40 promoter/enhancer sequences, and the biological implications of an RNA-based injectable like BNT162b2. Multiple advanced methodologies and carefully controlled experiments support these conclusions.
Using highly sensitive assays such as the Qubit dsDNA High Sensitivity Assay, Quant-iT PicoGreen dsDNA Assay, and AccuBlue High Sensitivity dsDNA Assay, researchers quantified the residual DNA content in the injectable lots. Their analyses revealed concentrations of double-stranded DNA (dsDNA) ranging from 32.7 to 43.4 nanograms per clinical dose—levels far exceeding the regulatory threshold of 10 nanograms per dose. The results were further validated through RNase treatment, which eliminated RNA interference and confirmed the accuracy of the DNA measurements.
The detection of SV40 promoter/enhancer sequences was achieved through Polymerase Chain Reaction (PCR) targeting specific genetic elements. Amplification of these sequences and visualization via gel electrophoresis provided conclusive evidence that the SV40 promoter/enhancer and other plasmid-derived sequences were present in all tested lots. The presence of these sequences underscores a manufacturing decision that raises significant safety concerns, particularly given the SV40 promoter’s established role in facilitating nuclear transport in mammalian cells.
To explore the biological implications of these findings, the study employed human HEK293 cells as a model system. Upon transfection with injectable material, these cells produced substantial quantities of spike protein, as quantified by ELISA. Remarkably, spike protein production persisted over several days, peaking on day five and remaining elevated on day seven. DNA isolated from the transfected cells confirmed the successful uptake of plasmid DNA, with the PCR amplifying plasmid-specific sequences from cellular DNA.
The study also uncovered evidence of extracellular vesicle-mediated secretion of spike proteins. Mass spectrometry revealed that spike proteins were predominantly secreted via exosomes, which serve as a mechanism for systemic distribution in vivo. The spike protein was found in high abundance in the extracellular vesicles, while minimal levels were detected in the surrounding soluble secretome. This raises critical questions about how these proteins may travel through the body and impact distant tissues.
Further analysis of the lipid nanoparticles used for injectable delivery revealed their role in encapsulating residual DNA. When the lipid nanoparticles were disrupted with Triton-X-100, significantly higher levels of DNA were released, demonstrating the incomplete removal of residual DNA during manufacturing. The DNA levels in treated samples were up to 6.7 times higher than in untreated ones, highlighting a crucial failure in production quality control.
The study’s findings were meticulously validated through reproducibility and the use of appropriate controls. Non-transfected cells served as negative controls, while plasmid DNA sequences known to be present were used as positive controls for PCR and other assays. The consistency of results across different DNA quantification methods and replicates further underscores the reliability of the conclusions.
These results confirm prior reports of residual DNA and its potential biological effects, including those by McKernan, Speicher, and Bulkhauts. This study corroborates and extends those findings by demonstrating prolonged spike protein production, the systemic transport of spike proteins via exosomes, and the presence of the SV40 promoter/enhancer sequence. The implications are profound, underscoring systemic shortcomings in manufacturing processes and regulatory oversight for RNA-based biologics. The findings demand immediate further investigation and re-evaluation of safety protocols to address these risks comprehensively.
This is not merely an academic concern. Regulatory agencies worldwide set stringent thresholds for residual DNA in injectable biological products to minimize risks such as oncogenesis or immune reactions. The study’s findings show that BNT162b2 failed to meet these thresholds and exposed a regulatory blind spot: RNA-based injections are held to standards designed for older technologies, ignoring the novel risks introduced by lipid nanoparticle delivery and modified mRNA platforms. Existing thresholds for residual DNA are defined for naked DNA (e.g. in insulin and other genetically produced drugs), but not for drugs containing nucleic acids packaged in transfection-competent components; regulatory bodies missed this important distinction.
The rush to deploy these injections cannot be disentangled from the broader context of the pandemic. Operation Warp Speed and its international counterparts have been hailed as triumphs of logistics and determination. But haste and innovation alone are insufficient to guarantee safety. The absence of robust, independent oversight of manufacturing processes was obvious then, and the effects are now made even more painfully obvious. This failure to ensure transparency and adherence to established safety standards represents a betrayal of public trust.
Furthermore, these revelations demand a reckoning with the regulatory frameworks that allowed this to occur. Why were these injected biologicals not subject to the same degree of scrutiny as traditional biological products? And why did international regulatory agencies fail to demand detailed disclosures about every component used in their production? Including a sequence like the SV40 “nuclear transport element,” undisclosed in approval documents, is more than a lapse—it is an affront to scientific transparency and accountability principles.
Looking ahead, the implications for public health are sobering. Trust in vaccination as a control measure for infectious diseases is already at an all-time low. Most public health professionals seem to think it is necessary for global health strategies. Studies like this, which reveal hidden risks and insufficient regulatory oversight, threaten to undermine confidence in not only COVID-19 injectables but also future vaccine technologies. The scientific community must confront these findings with honesty and resolve, prioritizing safety and transparency over expedience.
Regulatory agencies must reassess their oversight mechanisms for RNA-based technologies to restore confidence, placing demands on data presentation and transparency. A new framework is needed—one that acknowledges the complexities of lipid nanoparticle delivery systems, the potential for genomic interactions, and the long-term consequences of widespread deployment. Independent laboratories should be funded and empowered to verify manufacturing claims, and manufacturers must be held accountable for discrepancies.
This study should be a wake-up call. It exposes the cost of rushing innovation without adequate safeguards, a misstep that could have far-reaching consequences for individual health and public trust. The lesson is clear: the next phase of scientific innovation must be guided by caution, integrity, and an unwavering commitment to safety—and it should be focused on integrative pathways to wellness.
Citation:
Kämmerer U, Schulz V, Steger K. BioNTech RNA-Based COVID-19 Injections Contain Large Amounts Of Residual DNA Including An SV40 Promoter/Enhancer Sequence. Science, Public Health Policy and the Law. 2024 Dec 02; v5.2019-2024



Another solid study result which clearly implies for an immediate moratorium on this use of this mRNA technology
Why were these injected biologicals not subject to the same degree of scrutiny as traditional biological products?
Because they were deemed "countermeasures" by the DoD, which was responsible for the rollout of these bioweapons in US and subsequently world-wide.
See Sasha Latypova's brilliant investigative work on this subject on Substack.