Patient Attrition Bias Explains The Latest NEJM Ivermectin Study
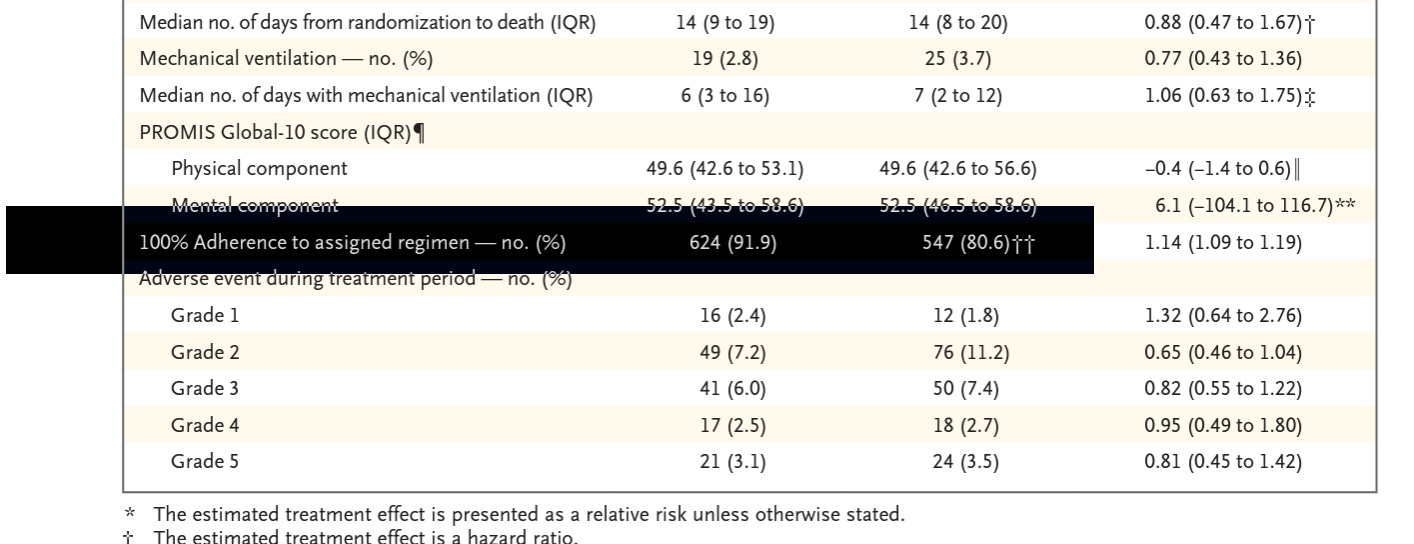
Their data show the number of people who stayed 100% on protocol. Guess which group didn't stay on protocol?
This:


Is not correct.
The study left out people who failed to adhere to the placebo treatment.
Check this out:
Both groups started with 679 people.
The Ivermectin group had 624 included after attrition.
For the per-protocol analysis, only 288 on placebo were still in the study.
Of the people who adhered to placebo with no benefit, we can expect they would have had more severe COVID-19 symptoms and sought treatment.
Note the patient attrition rate is far less in the Ivermectin group in the “100% Adherence to the assigned regime” analysis, but still biased toward people leaving the study to seek other treatment.
It’s a huge problem if more of one group is dropped from the study due to events that are clearly related to the outcomes measured.
The sickest people in the both groups would have started doing anything other than the pills they were given. The placebo group received visually identical pills in the identical packaging as the active drug, and they left in droves, whereas for the Ivermectin group, they clearly felt well enough to stay on the protocol.
Anyone can see that.
The paper is not to be taken seriously, and should be eliminated from future meta-analyses due to this obvious sign of bias.






They're so desperate to cover them themselves retroactively for blocking us from using Ivermectin that they'll publish anything. When I was training (a million years ago) we revered the New England Journal of Medicine, and Lancet. They were like gods to us. I don't know if they've just gone way down hill due to politics, or if they were always bad and we just didn't realize it at the time. It's really scary.
Within 7 days? Why so long? Why not within 24 hours?
I've known people who were ill with covid then got better by the 3rd day. By the time that person got treatment in this study they could have already been 4 days out from feeling better again.
"We have a new cancer drug! But we have to give it to people who have been in remission for a decade"
Uhh ok?