FDA Has No Bar to Lower on Bivalent Boosters: YOUR ACTION is Needed to Hold them to Standard
FDA cannot go any lower than to ignore the absence of legally required data from Pfizer on myocarditis
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) falls under Center for Biologics Evaluation and Research (CBER), which falls under the FDA, which falls under the HHS.
FDA has thrown out, once again, even any pretense of being serious about paying attention to data on vaccine safety and efficacy and appears ready to rubber stamp Pfizer’s bivalent booster.
Due to FDA’s reckless actions, Americans will now only be pressured/ cajoled/ manipulated/ coerced into accepting an annual unproven vaccine. Children and older Americans will be coerced into accepting two doses of bivalent shot, with the idea that perpetual boosters at unspecified time intervals will follow.
There are, however, a few massive flies in the ointment. First, there are no data that show that annual boosters decrease the risk of severe disease in healthy young adults and children.
Worse, it appears that Pfizer never produce the data they had to (as in were required to) produce on troponin levels resulting from their bivalent shot.
From FDA’s August 23, 2021 Approval Letter:
“We are issuing Department of Health and Human Services U.S. License No. 2229 to BioNTech Manufacturing GmbH, Mainz, Germany, under the provisions of section 351(a) of the PHS Act controlling the manufacture and sale of biological products. The license authorizes you to introduce or deliver for introduction into interstate commerce, those products for which your company has demonstrated compliance with establishment and product standards.
Under this license, you are authorized to manufacture the product, COVID-19 Vaccine, mRNA, which is indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older”
HOWEVER:
“We have determined that an analysis of spontaneous postmarketing (sic) adverse events (you) reported… will not be sufficient to assess known serious risks of myocarditis and pericarditis and identify an unexpected serious risk of subclinical myocarditis.”
The letter then gives the timelines and deadlines by which the data on subclinical myocarditis must be provided for the license to remain in good standing: some of these dates have passed, and there is no sign of such data from Pfizer.
Data from other sources tell us that the serious risk of subclinical myocarditis may be far higher - up to 100 times greater in those accepting the jab.
The FDA’s briefing document on Pfizer’s bivalent booster, however, does not mention of any data from Pfizer on this adverse event, instead includes this standard “nothing to see here” language:
“Within 28 days after any dose, unsolicited adverse events were reported by 30.7% of mRNA-1273.214 recipients, and generally represented illnesses and events typical of infancy/childhood. All were mild to moderate in intensity, except for one serious adverse event of asthma in a 5-year-old participant with onset 13 days after Dose 1, which was assessed as unrelated to (the) study (of the) vaccine by the investigators.”
When you see that last sentence (and we see it all the time in vaccine prospective clinical trials for “unsolicited” events), you have to wonder how they magically know the vaccine did not cause the event. We are supposed to use RCTs to determine the causality of these events, not a medical opinion. What DID cause the 5-year-old’s new-onset asthma, if not the vaccine? And what RCT was used to determine that causality?
Medical opinion is the second lowest level of scientific evidence on the causality of adverse events, as it is always debatable. The lowest form of scientific evidence is the medical opinion of investigators who are motivated to rule out any unsolicited adverse events in a trial of a vaccine that returns billions in revenue.
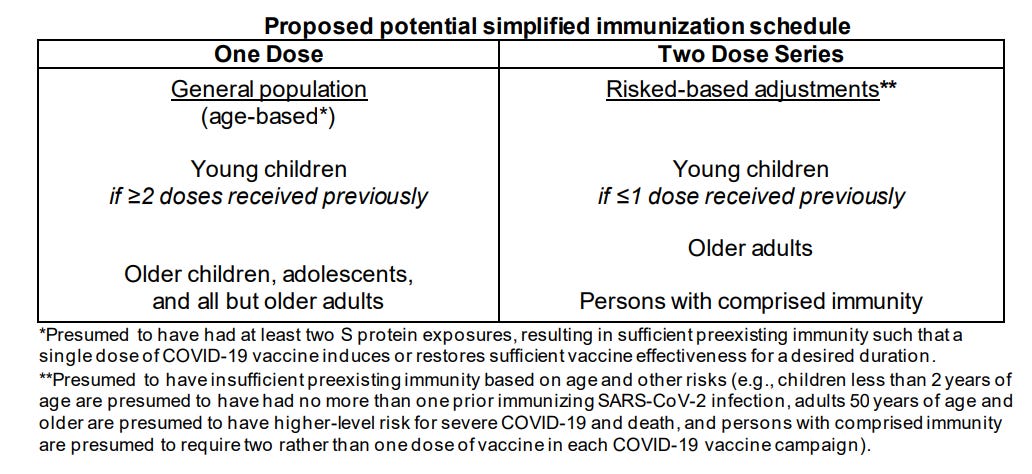
With not even a mention of the legally required data on myocarditis, VRBPAC plows ahead and proposes a schedule:
Remember, these repeated exposures are the reason for the concern over pathogenic priming. Why in the world when SARS-CoV-2 spike proteins are known to be toxic would the US FDA be hell-bent on ignoring the insufficiency of Pfizer’s filing of data?
Or do they have the data and merely decided not to bring it forward?
Either way, this is clearly on the FDA. This proves that FDA is captured by Pfizer and has zero interest in protecting the US public from harm from vaccines.
Whose children will suffer myocarditis as a result of FDA’s failure to provide translational oversight?
URGENT CALL TO ACTION - What You Can Do To Help: Contact your Congressional representatives and Senators and send them this article and demand censure of the FDA by the US House of Representatives an incorruptible civilian oversight board over HHS on vaccine safety (The Research Intelligence Network). With thousands of emails and phone calls, we’ll bring the lack of sufficient oversight by the FDA to the forefront of the ongoing issues and force them to do their jobs.






I've sent this message to both NJ senators and my Rep but in the last 3 years I've sent them all multiple letters and emails about vaccine safety, health freedom, etc and each time get back a canned response basically saying they won't do anything. So much for being "my" representative. Big Pharma / Agri and Insurance are all in their pockets.
The FOIA documents analyzed by Sasha Latypova indicate that FDA does not have the legal responsibility to approve the vaccines. It is merely play-acting at approving them. So unfortunately, this effort is pointed at the wrong agency. The real authority is BARDA, acting on behalf of DOD. https://rumble.com/v25fjoc-foia-documents-reveal-covid-pandemic-was-a-secret-dod-operation-dating-back.html