A Pediatrician Called Me And Asked Me What Would I Do?
He's running 30-40 tests per day. This is (in part) what I told him.
(Dear Reader: Twitter and I have entered into another staring context, so please share this across all social media channels. Much appreciate! -Dr Jack)
I have a friend who I respect very much, and the feeling is mutual. He’s an experienced pediatrician who is currently practicing and has seen many patients who have respiratory symptoms. He’s testing for COVID-19, but he knows something is not quite right. We’ve been comparing notes on vaccines for a long time. He called me yesterday, and he trusts my logic and my core ethos.
“OK, listen” he said. “I’m running 30-40 rapid antigen tests in the clinical every day. These kids have mild symptoms, none are going to the hospital, you know, it’s like, Omicron is just a mild cold. But we’re testing, and some are positive. People are, you know, quarantining and it’s disruptive and all I can really do is test. So what would you do?”
So here’s what I’d do.
Don’t test asymptomatic patients, but advise any who are concerned but asymptomatic on proper pre-COVID-19 maintenance nutrition (daily D, A, C, quercetin, zinc, occasional iodine, selenium). FDA’s doses on D are too low, especially for higher latitudes. Have them eat whole peeled citrus, not just supplements.
For symptomatic patients, run a panel of non-COVID-19 respiratory assays first to rule out COVID-19.
The rapid antigen test should be considered in light of other test results, symptoms and past exposures. Plus, be aware it may be detecting the common cold (more below).
Run a PCR test on patient if and only if they (a) are in a house or other intimate setting where there has been a recent (last 4 weeks) confirmed case of COVID-19, or (b) present with clear classical features of COVID-19 (loss of sense of smell & taste, fever).
Sample them twice, and send one sample to Dr. Sin Hang Lee in Millford, CT for confirmatory Sanger sequencing. His sequencing-based COVID-19 diagnostic assay can’t be fooled by false positives caused by the use of universal cycle thresholds. Plus now he’s added an Omicron-specific Sanger sequencing assay. (NB: I receive no funds from Dr. Lee or Milford Molecular Diagnostics, but IPAK is funding a research study by him).
Why This Makes Sense
With PCR, the CDC and FDA has us all fighting a raging bull elephant with an intellectual flyswatter. They appear to have no clue about the value of molecular diagnostic protocols, decision trees, or even conditional probability.
So, let’s break this down, here P(X) = “The Probability of Event X” and P(X|Y) means “The Probability of X given Y is true”. Similarly, P(X|Y,Z) = “The Probability of Event X given that Y and Z are both true”, and P(X|Y, Not Z) = “The Probability of Event X given that Y is true but Z is false”. Look what we can do, formally, even without any rate data:
P(COVID|Symptoms) > P(COVID|No Symptoms)
P(COVID|Symptoms, Exposure) > P(COVID|Symptoms)
P(COVID|Exposure) > P(COVID|No Exposure)
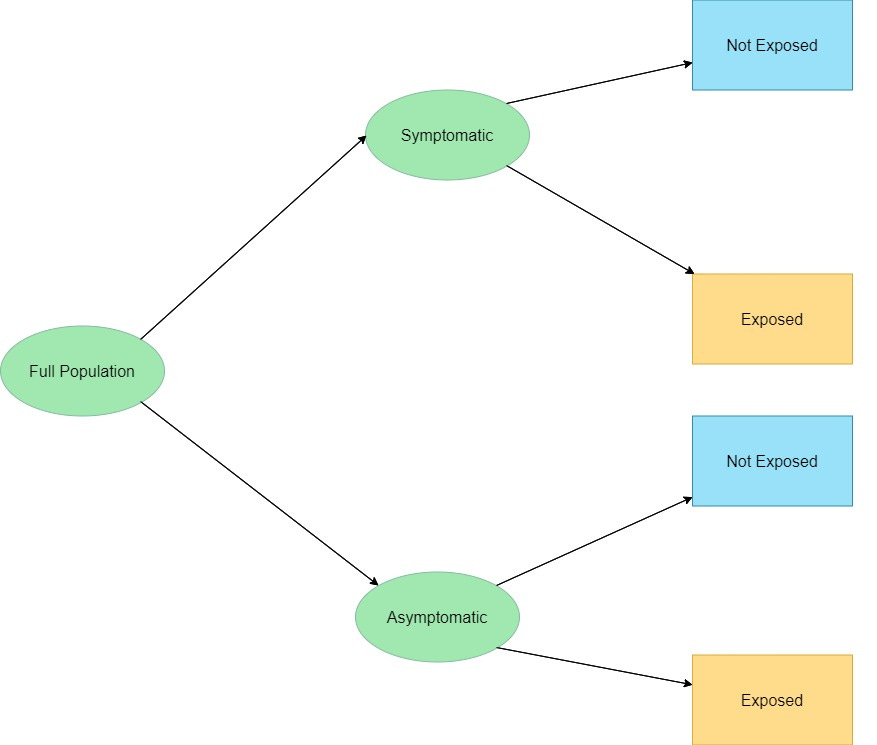
In decision tree framework, just this part looks like this:
At any given time during the year, the % Symptomatic and Asymptomatic will vary, but most people are asymptomatic at any given time. Exposure information can be gleaned from a pre-office visit patient interview.
The next step, in my view, should be a non-COVID-19 respiratory pathogen panel. Why?
P(COVID|Symptoms, Non-COVID Test +) > P(COVID|No Symptoms)
P(COVID|Symptoms, Non-COVID Test +, COVID Test +) > P(COVID|Symptoms, Non-COVID Test +)
Since COVID-19 has an extended prodromal period, the first node could arguable Exposed vs. Not Exposed, but since no clinical intervention occurs so far, it does not matter if which are asked first.
Clearly, the Asymptomatic, Not Exposed would be lowest priority for any testing.
Then, which of the two of (Symptomatic, Not Exposed) and (Asymptomatic Exposed) is higher priority for testing is an empirical question (data needed).
Nevertheless, with only these steps, the accuracy of any testing will be improved dramatically. The published false positive rates (ranging from 11% to conservatively 40%) will only apply to those patients who are tested. False negatives due to non-testing will be captured when the infected patients become symptomatic and their status changes.
The Role of Non-COVID Tests
The next node in the decision tree I would recommend is a panel of tests for non-COVID19 respiratory pathogens, such as Influenza A or B, Respiratory Syncytial Virus (RSV), Bacterial Pneumonia, and Pertussis. This is for two reasons.
First, people without COVID-19 might still test positive due either the to cross-reactivity of the antibody tests (some people find tap water to be positive!), or due to the use of high cycle threshold values (Ct) in the use of PCR testing. Dr. Lee estimates the false positive rate may be as high as 91% (data forthcoming).
Second, people with any non-COVID19 respiratory illness should receive care specific to that particular respiratory illness. This would be particularly important for pertussis and for bacterial pneumonia.
NB: COVID19 is a microvasculature disease, and the “pneumonia” is actually microclots in the lung. Thus, physicians should consider recommending a 4-10 day course of full dose aspirin to reduce the risk of severe COVID-19. (Yes, long-term use of full-dose aspirin is associated with stomach bleed. Four to ten days is not “long-term”.
Should Physicians Use Rapid Antigen Tests or PCR First?
CDC says
“It is important for healthcare providers and testing personnel to understand the performance characteristics, including sensitivity, specificity, and positive and negative predictive values, of the particular antigen test being used, and to follow the manufacturer’s instructions for use, which summarize performance characteristics. See FDA’s In Vitro Diagnostics EUAs for detailed information about specific authorized tests.”
Or at least they should. Looking at one of the popular tests, the Abbott BINAX rapid antigen, shows they tested for specificity using 3 samples each with human nasal swab material spike with various pathogens. They provided assurance of high specificity via BLAST algorithm of the SARS-CoV-2 antigen targeted by their antibody test, but MERS and HKU1 cannot be ruled out. HKU1 is a type of “the common cold”.
In this regard, the are correct. Specific rapid antigen test’s false positive rate can be known from the data submitted to FDA. CDC says
“If you are found to be infected with a common coronavirus (229E, NL63, OC43, and HKU1), that does not mean you are infected with the 2019 novel coronavirus.”
So the rapid antigen test could be the common cold. That means its result needs to be confirmed before clinical decisions are made on the base of testing.
That means, in an ideal world, that PCR testing will be conducted on all positive rapid antigen tests. But we also know that PCR has a false positive risk - and also a false negative risk. This means the general-used value of the Rapid Antigen testing on the symptomatic exposed would be to rule OUT COVID-19, not to RULE IN COVID-19. If the non-COVID pathogen assay includes HKU-1, the Rapid Antigen test becomes a “ruling in” diagnostic.
So, now that that’s clear up, what about PCR testing in the final group (Exposed, Symptomatic, Non-COVID-19 Test -, Rapid Assay Test +)?
While most persons tested will the COVID-19 positive, the false positive risk of the test is still a problem. Thus, Sanger sequencing should be adopted, or I should say returned as the Gold Standard. Why?
Because telling a person they have COVID-19 when they do not has consequences like quarantine and isolation, lost work, etc. But telling them they have COVID-19 when they do not also causes them to think they are immune due to the strong, broad, durable and deep immunity infection provides.
Which means they will let their guard down and very likely become a COVID-19 patient who refuses to believe they have COVID-19. So they won’t act fact toward early, aggressive ambulatory care.
That’s it in a nutshell. I hope this helps.
Do you agree? If yes, drop a comment. Better yet, if not, drop a comment and let me know why.
I’ll be working to formalize the probabilities with a decision tree using empirical estimates of the nodal probabilities to calculate the overall emergent performance evaluation measures like sensitivity, specificity, negative predictive value and positive predictive value.
If you’d like to support this type of research, you can become an IPAK Monthly Science Hero using this handy form. Or, considering upgrading to a paid subscriber to Popular Rationalism. Of give a gift subscription to your doctor. Either way, you’re driving me forward to rational approaches for molecular diagnosis of COVID-19 and SARS-CoV-2 infection.
1/3/2022 Press Release: Omicron, Delta or Other Covid Variants Can Be Diagnosed for Any Patient by Sanger Sequencing, Milford Molecular Diagnostics Dr. Sin Hang Lee Announced https://www.businesswire.com/news/home/20220103005028/en/
Here’s an example diagnostics paper not related to COVID-19 for those who want to learn:
Here’s my much earlier CDMS paper with Harry Shi -




Yes, I agree with your assessment. Our state health department sent out text messages to every phone to offer each person up to 8 free tests. It seems like an oversampling bias to encourage asymptomatic testing of an entire population.
Coincidently, there is a meeting this Wednesday to discuss vaccine mandates for children to attend public school and/or day care. I believe they are purposely inflating COVID case numbers to push through their proposed mandates. It's criminal!
Ambulatory care? Perhaps the first step is early treatment, no?