21st Century Cures Act and FDA's Codification: The Risks of "Minimal Risk" Waivers
Informed Consent is an Inviolable Basic Human Right. FDA's New Lax Rule on Informed Consent Could Compromise Public Health and Ethical Standards. IPAK will Require Informed Consent for All Studies.
PUBLIC NOTICE: CONTRA FDA, The IPAK-EDU IRB, established this year, will require informed consent despite investigators' appearances or opinions that the investigative procedures constitute “minimal risk.” We also call on all IRBs to hold investigators to appropriate standards regardless of FDA’s Congressionally approved and lawfully lax position.
The concept of informed consent in medical practice and research has evolved significantly over centuries, shaped by landmark legal cases, ethical codes, and regulatory policies.
Early Legal Precedents: Establishing the Need for Consent
The journey began in the 18th century with the case of Slater v. Baker and Stapleton (1767). Richard Slatter had fallen from his horse and broken his leg in several places. The leg healed ungracefully. In this case, surgeons performed a bone-setting procedure without the patient's consent, leading to a ruling in favor of the patient.
According to Miller, “The defendant surgeons were accused in court of disuniting (rebreaking) the callus (the temporary bony tissue that connects fractured bones) in Slatter’s recently healed broken leg. The court concluded that, without Slatter’s consent, they had rebroken his leg and that their motivation was probably to use a new device on the leg that would have constituted an experiment in the eyes of the court. At trial, an unidentified woman witness described the device as a ‘heavy steel thing that had teeth.’ The court expressed the reasons for the requirement of consent in surprisingly modern terms - ‘indeed it is reasonable that a patient should be told what is about to be done to him, that he may take courage and put himself in such a situation as to enable him to undergo the operation…’” SLATER V. BAKER AND STAPLETON (C.B. 1767): UNPUBLISHED MONOGRAPHS BY ROBERT D. MILLER.
This case established an early precedent that medical practitioners must obtain consent before performing procedures, highlighting the importance of patient autonomy and laying the groundwork for future developments in informed consent.
The principle of informed consent was further reinforced in the early 20th century with Mohr v. Williams (1905) and Pratt v. Davis (1905). In Mohr v. Williams, a surgeon operated on the patient's left ear instead of the right ear, as initially consented. The court found that the surgeon had violated the patient's rights by performing an unauthorized procedure. Similarly, in Pratt v. Davis, a surgeon performed a hysterectomy without explicit consent from the patient; the procedure was an experimental treatment for her epilepsy. Both cases emphasized the necessity of obtaining specific consent for medical procedures, reinforcing the legal requirement for informed consent and patient autonomy.
The Introduction of Informed Consent: Salgo v. Leland Stanford Jr. University Board of Trustees (1957)
The legal doctrine of informed consent was formally introduced in Salgo v. Leland Stanford Jr. University Board of Trustees (1957). In this case, the patient suffered complications from a diagnostic procedure without being fully informed of the risks. The court's ruling emphasized the necessity of informing patients about the risks involved in medical procedures, thus formally establishing the term "informed consent" in legal discourse. This landmark case underscored the importance of patient understanding and decision-making in medical practice.
Ethical Codes: Setting Standards for Human Research
The atrocities of World War II highlighted the need for ethical standards in medical research, leading to the development of the Nuremberg Code (1947). This code, formulated in response to the unethical medical experiments conducted during the war, laid down fundamental principles such as the voluntary consent of human subjects and the necessity to avoid unnecessary physical and mental suffering in experiments. The Nuremberg Code set the foundation for ethical standards in human experimentation and underscored the critical importance of voluntary consent.
Building on the principles of the Nuremberg Code, the Declaration of Helsinki (1964) was formulated by the World Medical Association to provide ethical guidelines for medical research involving human subjects. The Declaration emphasized respect for individuals and their right to make informed decisions, the necessity for research to be based on a thorough knowledge of scientific literature, and the requirement for research protocols to undergo review by an independent ethics committee. The Declaration of Helsinki set global standards for ethical principles in medical research, reinforcing the necessity of informed consent.
Addressing Ethical Breaches: The Tuskegee Syphilis Study and the Belmont Report
The infamous Tuskegee Syphilis Study (1932-1972), where untreated syphilis was studied in African American men without their informed consent, highlighted severe ethical breaches in research. The public outrage and ethical concerns arising from this study led to significant policy changes and increased oversight of research ethics. The study's fallout profoundly influenced the development of modern moral standards, underscoring the critical importance of informed consent and protecting research participants.
In response to the ethical breaches exemplified by the Tuskegee Study, the Belmont Report (1979) was developed to provide a framework for ethical conduct in research involving human subjects. The Belmont Report established three fundamental principles: information, Comprehension, and Voluntariness. It also helped develop the concepts of Beneficience. The Belmont Report's principles guide informed consent processes today, ensuring ethical conduct in research.
Modern Standards: Comprehensive Regulations for Ethical Research
Modern standards for informed consent are enshrined in comprehensive regulations such as 45 CFR 46 (Common Rule) and 21 CFR 50. The Common Rule, a federal policy for protecting human subjects in research, mandates Institutional Review Board (IRB) oversight, detailed requirements for obtaining informed consent, and additional protections for vulnerable populations. These regulations ensure robust informed consent procedures and participant protections in research.
Similarly, the FDA regulations on informed consent (21 CFR 50) provide precise requirements for informed consent documentation, special protections for children and other vulnerable populations in clinical investigations, and high standards of participant protection in FDA-regulated clinical trials. These regulations ensure rigorous informed consent processes and maintain ethical standards in medical research and practice.
The evolution of informed consent has been shaped by landmark legal cases, ethical codes, and regulatory policies over centuries. From early legal precedents requiring patient consent to comprehensive federal regulations, the history of informed consent reflects an ongoing commitment to upholding patient autonomy and ensuring ethical conduct in medical research. Each development has contributed to a framework that prioritizes the rights, safety, and well-being of research participants, ensuring that informed consent remains a cornerstone of ethical medical practice.
The 21st Century Cures Act (2016)
The 21st Century Cures Act passed in 2016, was widely celebrated as a monumental step in accelerating the discovery, development, and delivery of innovative medical treatments. Designed to streamline the regulatory process, the Act aimed to enhance funding for medical research, facilitate the approval of breakthrough therapies, and modernize clinical trials to ensure patients could access life-saving treatments more rapidly. Proponents highlighted its potential to drive significant advancements in biomedical research, reduce the burdens of chronic diseases, and improve overall public health outcomes.
However, despite the laudable goals and overwhelming bipartisan support, a critical provision was introduced at the last minute. On the eve of the Act's finalization, a clause allowing the waiver of informed consent for certain minimal-risk studies was quietly added. This historical fact raises substantial concerns about the potential for ethical lapses and the protection of patient rights in the pursuit of scientific progress.
We should all be safety advocates and industry watchdogs. This article aims to showcase the potential for abuse of the minimal risk waiver provision under the 21st Century Cures Act and offer solutions. This article will highlight specific vulnerabilities that may arise and propose measures to mitigate these risks, ensuring that patient safety and ethical standards remain paramount in clinical research.
Importance of Informed Consent in Clinical Research
Informed consent is a cornerstone of ethical medical practice and clinical research. It is a process through which a participant voluntarily confirms their willingness to participate in a particular study after having been informed of all aspects of the trial that are relevant to their decision-making (Breathe). This includes details about the purpose, duration, procedures, risks, benefits, and alternatives to participation. The requirement for informed consent respects the autonomy of individuals, allowing them to make knowledgeable decisions about their health and involvement in research. Historically, the lack of informed consent has led to grievous abuses, such as the Tuskegee Syphilis Study (Children’s Health Defense), underscoring its critical role in protecting patient rights and maintaining public trust in the medical community.
Historical Abuses by Pharmaceutical Companies
Case Study 1: Vioxx (Rofecoxib)
One of the most notorious examples of pharmaceutical misconduct involves Merck's painkiller, Vioxx (Rofecoxib). Introduced in 1999, Vioxx was initially hailed as a breakthrough for patients suffering from chronic pain. However, evidence soon emerged linking the drug to increased risks of heart attacks and strokes. Merck was found to have downplayed these cardiovascular risks in its marketing and communications, prioritizing profit over patient safety. In 2004, Vioxx was withdrawn from the market, and Merck faced significant legal repercussions, including a $4.85 billion settlement to resolve claims from affected patients (New York Times). This scandal also led to changes in regulatory practices, increasing scrutiny, and post-market surveillance of drugs (sometimes unofficially referred to as “Phase IV studies”.
Case Study 2: The Opioid Crisis
The opioid crisis serves as another stark reminder of the pharmaceutical industry's potential to cause widespread harm through misleading practices. Companies like Purdue Pharma aggressively marketed opioid medications, such as OxyContin, while downplaying their addictive potential. This deceptive marketing strategy contributed to an epidemic of opioid addiction and overdose deaths. Investigations revealed that these companies misled healthcare providers and the public about the safety and efficacy of opioids, leading to numerous lawsuits and substantial financial penalties. Purdue Pharma, for instance, pleaded guilty to criminal charges and faced billions in fines and settlements (CNN). The impact on individual lives, families, and communities has been devastating, prompting significant changes in prescription practices and increased regulation of pain management drugs.
Case Study 3: Avandia (Rosiglitazone)
GlaxoSmithKline's (GSK) handling of the diabetes drug Avandia (Rosiglitazone) is another example of pharmaceutical negligence. Studies indicated that Avandia increased the risk of heart attacks, yet GSK failed to disclose this information to the public and regulatory authorities. The company faced legal actions and agreed to a $3 billion settlement over claims of fraud and failure to report safety data accurately (Everyday Health). This case not only highlighted the need for transparency and accountability in the pharmaceutical industry but also led to stricter guidelines for reporting and managing drug safety data.
These cases illustrate a troubling pattern of behavior within the pharmaceutical industry, where profit motives can overshadow patient safety. The Vioxx scandal, the opioid crisis, and the Avandia controversy all underscore the importance of stringent regulatory oversight and the ethical imperative to prioritize patient well-being. These scandals have led to changes in regulatory policies, such as enhanced pharmacovigilance and more rigorous post-market surveillance, to prevent similar abuses. As we consider the implications of the 21st Century Cures Act's waiver provision (GovInfo.com), it is crucial to remember these lessons and ensure that similar abuses do not recur. Vigilance and continuous improvement in regulatory practices are essential to maintaining public trust and protecting patient safety.
Understanding the New FDA Rule
The 21st Century Cures Act introduced a provision allowing Institutional Review Boards (IRBs) to waive or alter informed consent requirements for certain minimal-risk studies (GovInfo.com). Codified in 21 C.F.R. § 50.22, this rule aims to streamline the research process by removing barriers that could delay important studies involving minimal risk to participants. Minimal risk is defined as the probability and magnitude of harm or discomfort anticipated in the research not being greater than those ordinarily encountered in daily life or during routine physical or psychological examinations. For example, studies involving the collection of non-invasive data, like surveys or routine clinical tests, might qualify.
The criteria for these waivers require that the research:
1. Involves no more than minimal risk to the subjects.
2. Could not practicably be carried out without the waiver or alteration.
3. Does not adversely affect the rights and welfare of the subjects.
It also requires that, whenever appropriate, the researchers provide the subjects with additional pertinent information after participation.
Intended Benefits
The primary goal of this provision is to facilitate the conduct of vital research that might otherwise be hindered by the complexities of obtaining informed consent. Other arguments for the 21st Century Cures Act - made nearly 10 years ago - included
Flexibility to expedite finding cures that were just around the corner.
Faster research at less cost.
The promise of individualized medicine.
Making it easier to find the “right patients for the right trials” (there are scientific abuses here that we will revisit in other articles).
Providing earlier access to new drugs with the promise of pharmacovigilance (Post-market surveillance).
Shorting the time from bench to bedside.
Preventing outsourcing of clinical research in the US to other countries.
By allowing for waivers in minimal risk scenarios, the 21st Century Cures Act was supposed to add to FDA’s already considerable flexibility in leveraging or relaxing policies with the aim of accelerating advancements in medical research and innovation. This was thought to be particularly beneficial for studies involving routine clinical practices or non-invasive procedures, where the “burden” (their language, not ours) of obtaining formal consent may outweigh the risks involved. For instance, large-scale epidemiological studies or quality improvement initiatives within healthcare settings can proceed more efficiently, potentially leading to quicker medical advancements and improved patient care.
Potential for Abuse
However, the “minimal risk” provision also opens the door to potential abuse, especially given the historical context of pharmaceutical companies prioritizing profits over patient safety. Financial relationships between IRBs and pharmaceutical companies could influence IRB decisions, leading to studies being misclassified as “minimal risk” to expedite approval and reduce costs, bypassing crucial informed consent protocols. The risk of misuse is particularly concerning in light of documented cases where financial incentives have compromised research integrity. For example, the undue influence seen in the Vioxx and opioid cases illustrates how financial interests can overshadow patient safety.
Past Incidents Tell Us Informed Consent is an Inviolable Basic Human Right
Reflecting on past incidents like the Vioxx scandal and the opioid crisis, it becomes evident how minimizing perceived risks can lead to significant public health issues. In these cases, the downplaying of adverse effects and the prioritization of market approval over patient safety had devastating consequences. The new FDA rule, while designed to enhance research efficiency, must be carefully monitored to prevent similar occurrences. Ensuring that the IRB's criteria for minimal risk are applied rigorously and transparently is essential to maintaining ethical standards.
Implementing stronger conflict-of-interest policies, increasing the transparency of IRB decisions, and enhancing regulatory oversight are crucial steps to mitigate these risks. Such measures can help ensure that the benefits of the new FDA rule are realized without compromising the ethical foundations of clinical research.
The Risks of “Minimal Risk”
IRB Loyalty to Sponsors
One of the primary risk factors associated with the waiver provision is the potential conflict of interest between pharmaceutical companies and IRBs. Pharmaceutical companies often fund the studies that IRBs oversee, and this financial relationship can influence the IRB’s decision-making process. There is a risk that IRBs might be more lenient in their evaluation of what constitutes a minimal risk to expedite the approval process and maintain favorable relations with their sponsors. Ensuring IRBs maintain independence and objectivity is crucial to safeguarding patient rights and earning public trust in clinical research. Implementing strict conflict-of-interest policies and requiring full disclosure of financial relationships are essential mitigate these risks.
Reliability of Adverse Event Reporting Systems
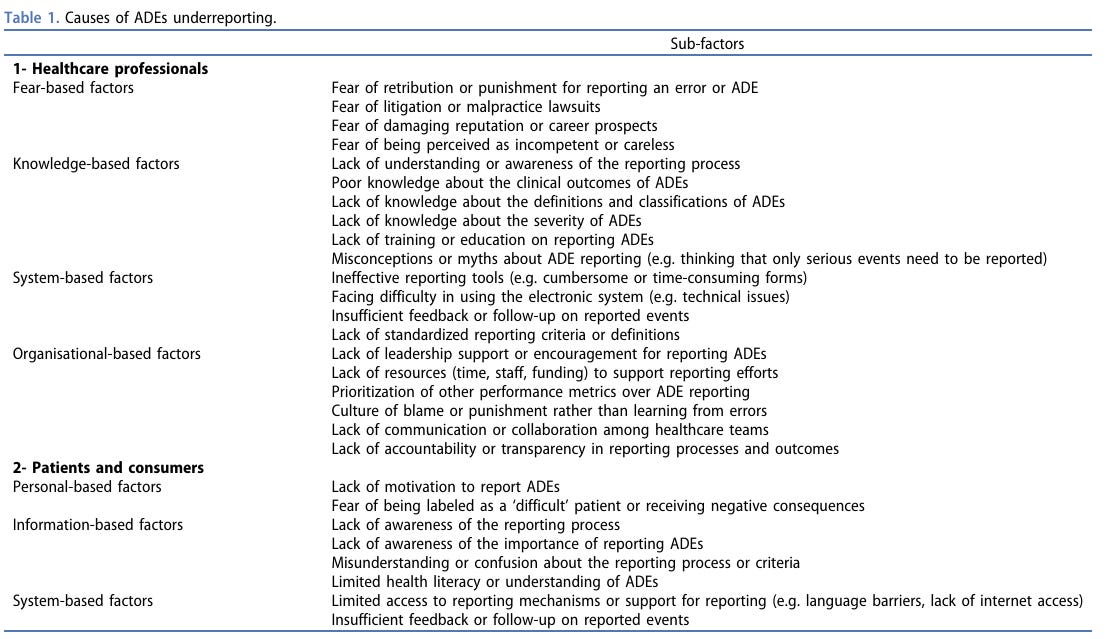
Adverse event reporting systems are critical for monitoring the safety of drugs post-market, but they have significant limitations. Passive systems, which rely on voluntary reporting by healthcare providers and patients, often suffer from underreporting and reporting bias. Many adverse events go unreported for many reasons (Table 1). This can result in incomplete data and a delayed response to potential safety issues, undermining the reliability of safety monitoring. Active surveillance systems, while more reliable, are resource-intensive and require robust infrastructure. These systems proactively collect data from electronic health records, insurance claims, and patient registries, providing a more comprehensive picture of drug safety. However, their implementation and maintenance pose significant challenges.
Table 1 is from Meslamani, 2023.
Ahmad Z. Al Meslamani (2023) Underreporting of Adverse Drug Events: a Look into the Extent, Causes, and Potential Solutions, Expert Opinion on Drug Safety, 22:5, 351-354, DOI: 10.1080/14740338.2023.2224558
Trust and Transparency in Adverse Event Reporting
The reliability of adverse event data also hinges on the trustworthiness of pharmaceutical companies and healthcare providers in reporting these events accurately and comprehensively. There have been instances where companies have failed to disclose critical safety information, as seen in the Vioxx and Avandia cases. Long-term safety data is particularly vulnerable to underreporting, as adverse effects may not become apparent until years after a drug's release. Ensuring transparency and accountability in adverse event reporting is crucial to safeguarding public health. Regulatory agencies must enforce stringent reporting requirements and conduct regular audits to verify the completeness and accuracy of reported data.
Monitoring and Enforcement
The effectiveness of the FDA's new rule hinges on robust monitoring and enforcement mechanisms. Regulatory bodies must conduct regular and rigorous audits of IRB activities to ensure compliance with ethical standards. Enhanced oversight is necessary to detect and promptly address any misuse of the waiver provision. This includes, for example, inspections and thorough reviews of the documentation supporting IRB decisions. This, however, presumes that the regulatory bodies themselves are not compromised by conflicts of interest, which we know is not valid. Ending regulatory capture will be necessary and healthy for safeguarding individual rights in clinical trials. Strengthening these mechanisms will help prevent potential abuses and maintain the integrity of the clinical research process. Ensuring that IRBs adhere strictly to the criteria for minimal risk as a priority, regardless of FDA’s lax position on assurances and promises of minimal risk, and that their firm decisions are transparent, defended in earnest, and well-documented is essential.
Specific Mechanisms to Influence IRB Decisions
The financial ties between pharmaceutical companies and IRBs can create subtle pressures that can influence IRB decisions. These pressures can manifest in various ways, such as through funding dependencies or future financial incentives. To mitigate these risks, it is essential to implement strict conflict-of-interest policies and ensure transparency in the decision-making process. This includes requiring IRBs to disclose financial relationships and recuse members with potential conflicts from related decisions. Such measures are crucial for preserving the objectivity and integrity of IRB reviews. Additionally, fostering a culture of ethical accountability within IRBs and among researchers is vital for upholding the standards of clinical research.
Mitigating the Risks of Minimal Risk
Strengthening IRB Independence
Promoting the independence of IRBs is essential to mitigate the risk of conflicts of interest. One effective strategy is to encourage the use of independent, third-party IRBs that have no financial ties to the pharmaceutical companies sponsoring the studies. Establishing guidelines that require the use of such independent IRBs, particularly for high-risk studies, can help ensure impartiality. Additionally, implementing strict conflict-of-interest policies and requiring IRB members to disclose any financial relationships with study sponsors are crucial steps. These measures can help maintain the objectivity and integrity of IRB decisions.
Enhancing Regulatory Oversight - After Controlling Regulatory Capture
Robust regulatory oversight is critical to ensuring compliance with ethical standards and preventing misuse of the waiver provision. Regulatory bodies like the FDA should increase the frequency and rigor of audits, focusing on IRB activities and decisions. Inspections and thorough reviews of documentation supporting IRB decisions can help detect and address any potential abuses promptly. Additionally, implementing centralized IRB reviews for high-risk studies, managed by regulatory authorities or independent entities, can provide an extra layer of oversight and ensure consistent application of ethical standards.
Improving Transparency and Accountability
Transparency and accountability in IRB decision-making processes are vital for maintaining public trust. Mandating public disclosure of IRB decisions, including the rationale for granting informed consent waivers, can enhance transparency. Requiring IRBs to publish detailed reports on their websites and involve multiple stakeholders, such as patient advocacy groups and independent experts, in the review process can ensure a more balanced and thorough evaluation.
Educational and Ethical Training
It is crucial to provide comprehensive training for IRB members on ethical standards, conflict of interest management, and the importance of maintaining independence. Developing mandatory training programs and continuous education requirements can help ensure that IRB members are well-informed and capable of upholding ethical standards. Establishing independent ethics committees to oversee IRB activities and ensure adherence to ethical guidelines can also provide an additional layer of oversight and accountability.
Leveraging Technological Solutions
Advanced technological solutions can play a significant role in enhancing the reliability and transparency of IRB activities. Implementing automated monitoring systems to track IRB decisions and flag potential conflicts of interest or unusual patterns can provide an additional layer of oversight. Utilizing blockchain technology to create an immutable record of IRB decisions and financial transactions can ensure transparency and accountability. These technological innovations can help prevent potential abuses and ensure that the benefits of the waiver provision are realized without compromising ethical standards.
Ensuring Reliable Adverse Event Reporting
Mandating active surveillance efforts is crucial to addressing the limitations of passive surveillance systems. This includes utilizing data from electronic health records, insurance claims, and patient registries to provide a comprehensive picture of drug safety. Strengthening reporting requirements and conducting regular audits to verify the completeness and accuracy of reported data can improve the reliability of adverse event monitoring. Encouraging transparency, accountability, and enforceability in adverse event reporting and ensuring that pharmaceutical companies and healthcare providers report long-term safety data accurately and comprehensively are essential to safeguarding public health.
Areas of Highest Risk and Liability
Psychotropic medications often carry significant risks and side effects, making them less likely to qualify as minimal risk studies. Similarly, invasive medical devices and high-risk drugs require stringent oversight to ensure patient safety. The history of pharmaceutical misconduct in these areas underscores the importance of maintaining rigorous informed consent protocols. Any relaxation of these protocols could have serious implications for patient welfare. Ensuring that high-risk studies undergo thorough and independent review is crucial to protecting participants.
High-Risk Drug Trials
One of the areas with the highest risk and liability concerns involves clinical trials for high-risk drugs. These are medications that have significant potential side effects or are intended to treat serious conditions, such as cancer therapies, psychiatric medications, or novel biologics. The inherent risks associated with these drugs necessitate rigorous oversight and comprehensive informed consent processes. Waiving informed consent in these scenarios could lead to severe adverse events and expose both patients and sponsors to significant harm and liability. For instance, the issues surrounding the antidepressant Paxil (paroxetine) in adolescents highlighted the critical need for stringent oversight in high-risk drug trials.
Paxil (Paroxetine), an SSRI approved by the FDA in 1992, has been widely used to treat various mental health conditions, including depression and anxiety disorders. However, its use has been marred by significant controversies and legal challenges. GlaxoSmithKline (GSK), the manufacturer, faced accusations of withholding critical data on Paxil's safety and efficacy, particularly regarding its use in adolescents. A notable instance is Study 329, which originally misrepresented Paxil as safe and effective for teens.
However, a re-analysis published in The BMJ revealed that neither Paxil nor imipramine showed statistically or clinically significant differences from placebo in treating depression. Furthermore, the re-analysis found that the paroxetine group experienced increased suicidal ideation and behavior, while the imipramine group had more cardiovascular problems. The FDA responded by mandating a black box warning on the drug.
In 2012, GSK settled with the U.S. government for $3 billion over charges including the unlawful promotion of Paxil for unapproved uses. These issues have highlighted the need for greater transparency and regulatory oversight in drug approval processes
Invasive Medical Device Studies
Clinical trials involving invasive medical devices, such as implants or surgical tools, represent another high-risk area. These devices often require surgical procedures for implantation or use, which carry inherent risks of complications, infections, and long-term health effects. The complexity and potential dangers of these devices mean that waiving informed consent could result in patients being exposed to substantial harm without their full understanding or agreement, increasing the liability for both researchers and manufacturers. The controversy surrounding metal-on-metal hip implants, which led to numerous complications and subsequent legal actions, underscores the importance of comprehensive informed consent in these trials (FDA).
Pediatric and Vulnerable Populations
Studies involving pediatric populations or other vulnerable groups, such as those with cognitive impairments or chronic illnesses, are susceptible. These participants are often unable to fully understand the risks and benefits of a study, making informed consent especially critical. Misusing the waiver provision in these contexts could lead to ethical breaches and significant liability. The case of the experimental HIV drug trials on foster children in the early 2000s, which lacked proper consent procedures, highlights the severe implications of inadequate protections for vulnerable populations (Vera Institute of Justice).
Long-Term Safety of Drugs, Devices and Vaccines
Research that focuses on the long-term safety of drugs or devices poses a unique set of challenges and risks. Long-term studies are essential to understanding the full spectrum of potential adverse effects that may not be apparent in short-term trials. Relying on passive surveillance and waiving informed consent in these studies can lead to underreporting of adverse events and an incomplete understanding of the long-term risks. This can have serious implications for patient safety and result in substantial liability for failing to adequately monitor and report long-term safety data.
An illustrative example is the reliance on the Vaccine Adverse Event Reporting System (VAERS) for long-term vaccine safety monitoring. VAERS is a passive surveillance system that depends on voluntary reporting, often leading to significant underreporting and incomplete data. Additionally, retrospective, population-wide, long-term vaccine risk studies routinely proceed without informed permission from parents, further complicating the ethical landscape.
Post-Market Surveillance Failures
Once a drug or device is approved and enters the market, continuous monitoring of its safety profile through post-market surveillance is critical. However, relying heavily on passive surveillance systems like VAERS can lead to significant gaps in safety data. If adverse events are underreported or not promptly addressed, patients can suffer harm, leading to legal repercussions for manufacturers and regulatory bodies. Ensuring robust post-market surveillance and active monitoring is crucial to mitigate these risks and liabilities. The issues faced with the anti-inflammatory drug Vioxx, which was linked to increased heart attack risk post-approval, highlight the importance of enforcement of post-market surveillance and the use of long-term outcome periods in clinical trials.
Financial Conflicts of Interest
Financial relationships between IRBs and pharmaceutical companies can introduce bias and influence decision-making processes, leading to ethical lapses and increased liability. If an IRB is perceived to be unduly influenced by financial incentives, its credibility and the integrity of its reviews can be called into question. This can result in legal challenges and damage the reputation of the IRB and the sponsoring companies. Implementing stringent conflict-of-interest policies and ensuring transparency in financial dealings are essential to maintaining trust and reducing liability risks.
Ethical Breaches in Study Design and Execution and Vulnerable Populations
Poor study design or execution that fails to adequately protect participants' rights and welfare can lead to serious ethical breaches and liability. Studies that do not follow ethical guidelines, fail to provide necessary information to participants, or improperly waive informed consent can result in harm to participants and significant legal consequences. Ensuring that all studies are designed and conducted in strict accordance with ethical standards is critical to mitigating these risks. The Tuskegee Syphilis Study, which notoriously failed to inform participants and resulted in severe ethical violations, remains a poignant reminder of the consequences of poor study design and ethical breaches. Informed consent must be built into the study design, and every person involved in the execution of the study, including the participants of the study, must understand the high priority of free, prior, and fully informed consent.
Achieving this can be challenging and costly, especially for vulnerable populations like children and minorities. However, arguing that it is imperative to expedite the rate of discovery at the cost of the accuracy and precision of the knowledge of risks and benefits is, in the final analysis, the same as cynically putting a price on the health, well-being, and lives of clinical trial participants—someone else’s life—in the name of profit and progress.
Drugs with Acute Health Risks if Failed
Certain drugs, if they fail, can lead to acute conditions that pose immediate and serious health risks, potentially leading to severe illness or death. These include blood pressure medications, heart medications, and powerful psychotropic drugs. The failure of such drugs can cause rapid deterioration in a patient's condition, making the need for rigorous informed consent and careful monitoring even more critical. For example, the improper administration or failure of blood pressure medications can lead to hypertensive crises, strokes, or heart attacks. Similarly, heart medications that fail can result in life-threatening arrhythmias or heart failure.
Powerful psychotropic drugs, if misused or if they fail to work as intended, can lead to severe mental health crises, including suicidal ideation or severe depression. For instance, the use of SSRIs (selective serotonin reuptake inhibitors) has been linked to an increased risk of suicidal thoughts and behaviors, particularly in young people. A meta-analysis of randomized placebo-controlled studies found a modestly increased risk of suicidality associated with antidepressants in pediatric patients (Psychiatric Times). Moreover, during the COVID-19 lockdown, a study showed that individuals consuming psychotropic drugs, such as tranquilizers and sedatives, exhibited significant rates of depression and suicidal ideation (International Journal of Mental Health and Addiction).
The complexities of psychotropic medications, including their side effects and the potential for psychological symptoms like anxiety and suicidal ideation, underscore the importance of careful monitoring and comprehensive treatment plans (Psychology Today) (BioMed Central).
These findings highlight the critical need for rigorous oversight and patient adherence to prescribed treatments to mitigate severe adverse outcomes. Ensuring these studies are conducted with the highest ethical standards and complete informed consent is essential to prevent such critical health risks.
Wrapping Up
In reviewing the provisions of the 21st Century Cures Act, particularly the waiver for minimal risk studies, it is clear that while the intent is to streamline and expedite important research, the potential for abuse remains significant. Historical abuses by pharmaceutical companies, such as those seen with Vioxx, the opioid crisis, and Avandia, underscore the importance of maintaining stringent oversight and ethical standards. The risks associated with passive surveillance systems, financial conflicts of interest, and inadequate long-term safety monitoring highlight the need for robust regulatory frameworks and transparent practices that the public can count on.
Call to Action to All IRBs: Require Informed Consent and Informed Permission in the Following Areas and Types of Studies Each Time
As we are all safety advocates and industry watchdogs, it is imperative to push for stronger protections to ensure that the waiver provision is not misused. Institutional Review Boards (IRBs) must take a proactive stance in requiring informed consent and informed permission in the following critical areas, as mandated by the Common Rule (45 CFR 46) and FDA regulations (21 CFR 50):
1. High-Risk Drug Trials: Trials involving medications with significant potential side effects or those intended to treat serious conditions, such as cancer therapies and psychiatric medications, and others discussed previously.
2. Invasive Medical Device Studies: Studies involving devices that require surgical implantation or use, such as implants and surgical tools.
3. Pediatric and Vulnerable Populations: Research involving children, cognitively impaired individuals, or those with chronic illnesses.
4. Long-Term Safety Studies: Studies that monitor the long-term safety of drugs, devices, and vaccines, particularly those relying on passive surveillance systems.
5. Post-Market Surveillance: Enforced Active Surveillance: Ongoing monitoring of approved drugs and devices to detect and address adverse events promptly - with severe penalties for non-compliance.
6. Studies with Financial Conflicts of Interest: Research funded by sponsors with financial ties to the IRBs or those with potential conflicts of interest.
7. Ethically Complex Studies: Research involving complex ethical considerations where the risks and benefits need careful and transparent evaluation. The use of independent ethics panels with no stake in the game would enhance the detection of problems and potential solutions
By mandating informed consent and permission in these areas, IRBs can uphold ethical standards, protect participant rights, and ensure public trust in clinical research.
Ensuring Ethical Standards
To prevent the recurrence of past abuses and to earn public trust, it is essential to uphold the highest ethical standards in clinical research. This involves rigorous training for IRB members, stringent conflict-of-interest policies, and advanced technological solutions to monitor and document IRB activities. Examples of successful implementation include institutions that have adopted blockchain technology for transparency and automated monitoring systems to flag potential conflicts. Fostering a culture of ethical accountability and continuous improvement is vital for upholding these standards. Adhering to the Common Rule (45 CFR 46) and FDA regulations (21 CFR 50) is paramount.
Looking Forward
Moving forward, the medical and research communities must remain vigilant and proactive in addressing the challenges posed by the waiver provision. As the minimal risk clause only empowers the FDA to allow the use of the minimal risk clause, individual IRBs can state their own, more ethical policies. The IPAK Institutional Review Board (IPAK-EDU IRB) will issue no waivers in return for promises of minimal risk. IRBs that do issue waivers in return for investigators' pledges of minimal risk should be considered susceptible to future litigation when participants sue. Courts will, in some cases, find that the risks a) were not minimal and b) the IRB could have known or should have sought more evidence behind safety assurances.
Continuous dialogue, policy development, and collaboration among all stakeholders—including regulatory bodies, researchers, patient advocacy groups, and the public—are crucial. Together, we can create a research environment that prioritizes patient safety, ethical standards, and the advancement of medical knowledge for the benefit of all. Special attention must be given to protecting vulnerable populations and adhering to the Common Rule, which provides a robust framework for the ethical conduct of research.
Enforcing Mandates for Protecting Vulnerable Populations
The protection of vulnerable populations in clinical research is non-negotiable. Adhering to the Common Rule (45 CFR 46), which outlines strict guidelines for the ethical treatment of research participants, is essential. This includes obtaining informed consent, ensuring that the risks are minimized and reasonable, and providing additional safeguards for those unable to consent themselves. The responsibility to uphold these principles lies with all stakeholders in the research community, ensuring that every participant's rights and welfare are protected.
Take Medical Ethics, Informed Consent and Human Rights at IPAK-EDU!
REFERENCES
Bazzano LA, Durant J, Brantley PR. A Modern History of Informed Consent and the Role of Key Information. Ochsner J. 2021 Spring;21(1):81-85. doi: 10.31486/toj.19.0105. PMID: 33828429; PMCID: PMC7993430. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7993430/






A valuable essay. It should be mentioned that the tort of battery is a separate cause of action from breach of the duty to obtain informed consent in the tort of negligence. Battery arises when the nature of the thing done which invades bodily integrity is different in nature from what was consented to be done. Battery has certain advantages for the plaintiff over negligence. Arguably, the COVID-19 gene therapy shots were not only in wholesale breach of requirements for informed consent, they also constituted batteries, given the presence of more than minimal quantities of DNA and other contaminants. in other words, the shots were not in nature vaccines, they were something essentially different. One test of this is, who would have agreed to receive the shots if they knew what was in them?
Looking for effective drugs in evil, Godless, for profit, big pharma's 60 billion in fraud and corruption fines world is a job for fools and suckers! Don't you know Big Pharma's [so-called] "medicine" is a or, the leading cause of death in the USA? Killing between 12.5 to 40 million in just the last 50 years, [Not counting their coved kills!] (According to the Johns Hopkins [low-ball] iatrogenic study or the well-documented book, Death by Medicine by Dr. Null?)
One might as well trust Satan, on how to get to Heaven!